Tulipalin A: Allergenicity, Detection, and Anti-Inflammatory Mechanisms
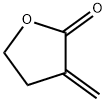
Tulipalin A (o.-methylene- y-butyrolactone)is the main sensitizer in tulips and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria). In a 1996 study,18%(9/51)of tulip workers were found to be allergic to tulipalin A. In a more recent study of 164 tulip workers,48(29.3%)had clinical evidence of contact dermatitis and subsequently underwent patch testing 17(35.4%)showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%])and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%)workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen. Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Tulipalin A induced phytotoxicity
Tulipalin A induced phytotoxicity, also Tulip Fingers and Alstroemeria dermatitis, is a common occupational allergy in floral workers exposed to Tulip and Alstroemeria cultivars. Similar phytotoxicity results from allyl isothiocyanate compounds present in the Cruciferae plant family. Both compounds were evaluated along with 240+ chemicals for inclusion on the ‘Allergen List’ compiled by Germany's Federal Institute for Risk Assessment (BfR). They were assigned to Category B: Solid-based indication for contact allergenic effects, because there is evidence of contact allergenic effects in animals and humans as well as induction of cross reactions in humans. In this article we highlight the importance of comprehensive patient histories in a case of Tulipalin A induced phytotoxicity misdiagnosed as a tinea manuum infection in an individual with suspected occupational fungal exposure and a review of the relevant scientific literature necessary to evaluate risk factors for the disease.[1]
Effects of tulipalin A exposure may be immediate or delayed. Manifestations of phototoxicity include pruritus and edema in the fingers and along the palmar surface of the hand. Lesions develop and gradually exfoliate. Repeated exposures may lead to significant thinning of the skin and pigmentation changes. Paronychia is commonly seen along with nail splitting and ulceration of the nail bed in more severe cases. Patients typically report significant pain associated with the outbreaks. Sensitive individuals may also develop rhinitis following airborne exposures. Differentiating tulipalin A induced phytotoxicity from the innumerable other dermatoses makes diagnosis difficult. A suspected case of contact dermatitis requires meticulous history taking and physical examination. Typically, a sensitized individual will develop acute symptoms 12-24 h after any subsequent exposures. Given the occupational history of this patient, a diagnosis of tulipalin A phytotoxicity is far more appropriate than tinea manis or Cryptococcus.
Rapid detection of Tulipalin A with SESI-Orbitrap MS
Alstroemeria and Tulipa cultivars, members of the Liliaceae order, are noteworthy cut flower species in the Netherlands. Although Tulipalin A is the primary cause of allergic contact dermatitis linked with Alstroemeria and Tulipa, little is known about its mechanism of action in both humans and microbes. The state-of-the-art sensitive secondary electrospray ionization coupled Orbitrap mass spectrometry system (SESI-Orbitrap MS) has emerged as a leading methodology for real-time monitoring of (semi-) volatile organic compounds (VOCs), primarily utilized in the medical field for breath gas analysis. In this study, a sensitive SESI-Orbitrap MS procedure is developed to online monitor tulipalin A release across plant organs and genera with minimal sample preparation.[2]
In conclusion, this study demonstrates that secondary electrospray ionization coupled Orbitrap mass spectrometry (SESI-Orbitrap MS) facilitates detection and tracking of tulipalin A release across plant organs and outline its cross-species distribution. Despite significant release across these flowers, limited signal intensities of tulipalin A are detected, likely below the disease phenotype threshold. Alternative defense mechanisms, such as the formation of raphides, are anticipated for these spring flowers. The species diversity analyzed here underscores tulipalin A biosynthesis within the Liliales order. By examining tulipalin A release from up to 17 distinct spring flowers, we noticed a significant tulipalin A release from Rosa, Gerbera, Neapolitanum, Ranunculus, Othocalis, Muscari, Galanthus, Tulipa and Alstroemeria. Tulipalin A was predominantly released from the Tulipa and Alstroemeria species, both belonging to the Liliales order, as stated in previous clinical and research studies.
Tulipalin A suppressed the pro-inflammatory polarization
Tulipalin A, also named α-methylene-γ-butyrolactone, is a naturally occurring small molecule extracted from Tulipa flowers, characterized by the presence of α- and β-unsaturated ketones. It serves as both a commercially available reagent with well-established chemical synthesis methods and a prevalent functional group found in anti-inflammatory sesquiterpene lactones. Tulipalin A suppressed the pro-inflammatory polarization of M1 macrophage via directly targeting NF-κB p65 and inhibiting its DNA binding activity in the nucleus.[3]
The NF-κB pathway represents a canonical proinflammatory signaling cascade that governs the polarization of M1-type macrophages. Therefore, we investigated the regulatory effects of Tulipalin A on NF-κB. Unexpectedly, unlike the NF-κB inhibitor sulfasalazine that impedes the nuclear translocation of NF-κB by inhibiting IκBα degradation Tulipalin A did not affect the nuclear translocation of NF-κB p65. Specifically, helenalin suppressed the NF-κB activation by covalently modifying the sulfhydryl group on the cysteine residue of p65 through the α-M-γ-B functional group. Inspired by Helenalin's targeting mechanism of NF-κB p65, we demonstrated that Tulipalin A inhibits the DBA of NF-κB by directly targeting the cysteine residue of NF-κB p65. To fully demonstrate the therapeutical effects of Tulipalin A on ALI, more in-depth studies, including evaluating the therapeutic effects of administering the drug after LPS-induced ALI, are worth implementing in future research work.
References
[1]McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014 Apr;4(2):181-3. doi: 10.4103/2229-5151.134187. PMID: 25024947; PMCID: PMC4093970.
[2]Phan ANT, Eerlings R, Mengers HG, Blank LM. Rapid detection of Tulipalin A with SESI-Orbitrap MS: an exploration across spring flowers. Plant Methods. 2025 Feb 5;21(1):14. doi: 10.1186/s13007-025-01331-6. PMID: 39910633; PMCID: PMC11795999.
[3]Linghu, Ke-Gang et al. “Tulipalin A suppressed the pro-inflammatory polarization of M1 macrophage and mitigated the acute lung injury in mice via interference DNA binding activity of NF-κB.” European journal of pharmacology vol. 984 (2024): 177034. doi:10.1016/j.ejphar.2024.177034
You may like
See also
Lastest Price from Tulipalin A manufacturers

US $0.00/KG2025-08-27
- CAS:
- 547-65-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $0.00-0.00/kg2025-04-18
- CAS:
- 547-65-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000