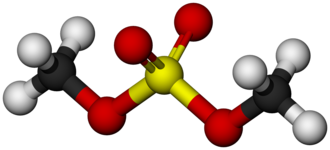
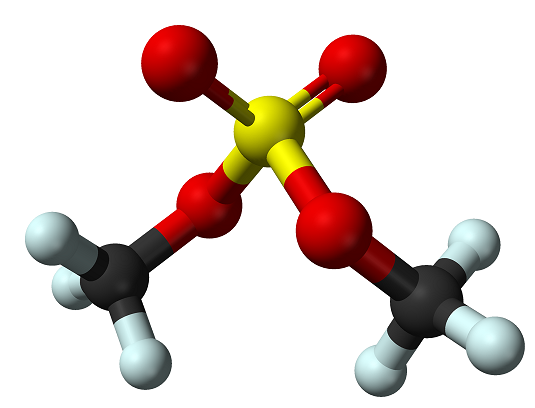
Uses of Dimethyl sulfate
Dimethyl sulfate is a colorless, oily liquid with a faint, onionlike odor. It is soluble in water, ether, dioxane, acetone, benzene, and other aromatic hydrocarbons, miscible with ethanol, and sparingly soluble in carbon disulfide. It is stable under normal temperatures and pressures, but hydrolyzes rapidly in water at or above 18 ℃.
Production method
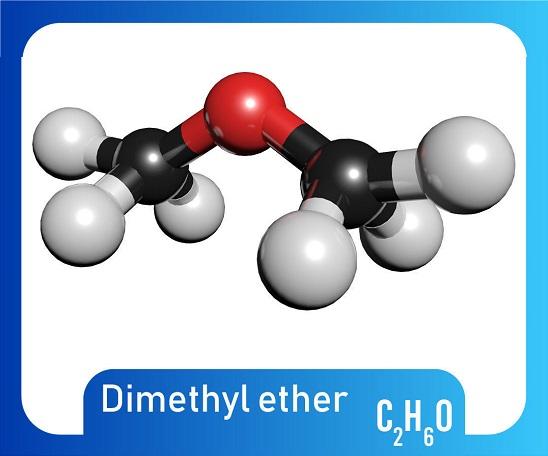
Dimethyl sulfate has been produced commercially since at least the 1920s. One production method is continuous reaction of dimethyl ether with sulfur trioxide. In 2009, dimethyl sulfate was produced by 33 manufacturers worldwide, including 1 in the United States, 14 in China, 5 in India, 5 in Europe, 6 in East Asia, and 2 in Mexico, and was available from 44 suppliers, including 16 US suppliers. There are no data on US imports or exports of dimethyl sulfate. Reports filed from 1986 through 2002 under the US Environmental Protection Agency’s Toxic Substances Control Act Inventory Update Rule indicate that US production plus imports of dimethyl sulfate totaled 10–50 million pounds. The simplest way of synthesizing dimethyl sulfate is by esterification of sulfuric acid with methanol as follows:
2CH3OH+ H2SO4→(CH3)2SO4 + 2H2O
Uses
The major use of dimethyl sulfate is as an alkylating agent. It is used in the manufacture of dyes, pharmaceuticals, and perfumes and in the extraction of aromatic hydrocarbons as a solvent. It is also used as a sulfonating agent. In World War I, it was used as a war gas. Dimethyl sulfate is used primarily as a methylating agent to convert compounds such as phenols, amines, and thiols to the corresponding methyl derivatives.
Compared to other methylating agents, dimethyl sulfate is preferred by the industry because of its low cost and high reactivity. It is also used as a methylating or sulfating agent in the manufacture of methyl esters, ethers, and amines in dyes, drugs, perfumes, pesticides, phenol derivatives, fabric softeners, polyurethane-based adhesives, and other organic chemicals. Dimethyl sulfate is also used as a solvent for the separation of mineral oils, for the analysis of auto fluids, and with boron to stabilize liquid sulfur trioxide.
Environmental Fate
Dimethyl sulfate is rapidly absorbed by ingestion, by inhalation, and through intact skin. It is slowly metabolized to methanol and sulfuric acid. Studies with dimethyl sulfate have shown that the lungs and brain exhibit a much higher degree of nucleic acid alkylation than the liver and kidneys. Since the lungs and brain receive a relatively larger proportion of the cardiac output, it has been proposed that dimethyl sulfate does not equilibrate throughout the body but breaks down in the organs that it penetrates first, owing to its alkylating abilities. The associated kidney damage suggests that dimethyl sulfate may be eliminated by the renal route.
You may like
Related articles And Qustion
Lastest Price from Dimethyl sulfate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 77-78-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-03-07
- CAS:
- 77-78-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons