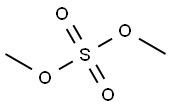
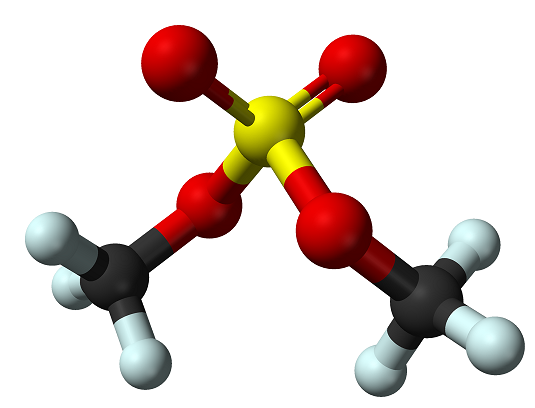
What is Dimethyl sulfate?
Dimethyl sulfate is a colorless oily liquid, odorless to a faint onion-like odor. It is very toxic by inhalation. It is a combustible liquid and has a flash point of 182°F. It is slightly soluble in water and decomposed by water to give sulfuric acid with evolution of heat. It is corrosive to metals and tissue. It is a potent methylating agent.

History
Dimethyl sulfate was discovered in the early 19th century in an impure form. J. P. Claesson later extensively studied its preparation. It was used in chemical warfare in WWI.
Production
Dimethyl sulfate can be synthesized in the laboratory by many different methods, the simplest being the esterification of sulfuric acid with methanol:
2 CH3OH + H2SO4 → (CH3)2SO4 + 2 H2O
Another possible synthesis involves distillation of methyl hydrogen sulfate:
2 CH3HSO4 → H2SO4 + (CH3)2SO4
Methyl nitrite and methyl chlorosulfonate also result in dimethyl sulfate:
CH3ONO + (CH3)OSO2Cl → (CH3)2SO4 + NOCl
Me2SO4 has been produced commercially since the 1920s. A common process is the continuous reaction of dimethyl ether with sulfur trioxide.
(CH3)2O + SO3 → (CH3)2SO4
Uses
Dimethyl sulfate is best known as a reagent for the methylation of phenols, amines, and thiols. One methyl group is transferred more quickly than the second. Methyl transfer is assumed to occur via an SN2 reaction. Compared to other methylating agents, dimethyl sulfate is preferred by the industry because of its low cost and high reactivity.
Related articles And Qustion
Lastest Price from Dimethyl sulfate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 77-78-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-03-07
- CAS:
- 77-78-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons