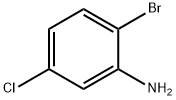
Uses of 2-Bromo-5-chloroaniline as a synthetic intermediate
2-Bromo-5-chloroaniline(2-BROMO-5-CHLOROBENZENAMINE; 2-BROMO-5- CHLOROANILINE; 2-Bromo-5-chloroaniline 97%; BenzenaMine, 2-broMo-5-chloro-; 2-BroMo-5-chloroaniline, 97%; C6H5BrClN)[1] is a commom synthetic intermediate or materials used in pharmaceutical synthesis and other drug materials.
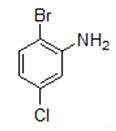
2-Bromo-5-chloroaniline can be used for synthesis of 2-bromo-5-chlorobenzenethiol by substitution reaction and reduction reaction in 1-, and the obtained 2-bromo-5-chlorobenzenethiol can further apply to prepare 2-bromo-5-chlorobenzenethiol benzothiadiazepine and benzothiadiazocine compounds[2], or react with the substituted aminopyrimidine to produce pyrimidinium chlorides[3]. 2-Bromo-5-chloroaniline was coupled with 3-(3-Chlorophenyl)-2-(3- methoxyphenyl) propanoic acyl chloride producing amide by acidylation, which will produce chiral 3-aryl-3-benzyloxindole with antitumor activity by further reaction[4].
By acetylation, 2-bromo-5-chloroaniline also can react with CO2 in the presence of DBU produing unsymmetrical ureas N-(2-Bromo-5-chlorophenyl)morpholine-4-carboxamide[5]. Besides, allylated anilides from 2-bromo-5-chloroaniline can react with dimethyl phosphonate and produce 3-phosphonoalkyl indolines[6]. 2-Bromo-5-chloroaniline in anhydrous dichloroethane coupled with thiophosgene in presence of sodium carbonate could produce 2-bromo-5-chlorophenyl isothiothianate, which could further apply to synthesize compounds for inhibiting c-myc/max/DNA complex formation [7]. Thus, 1-NH2 of 2-bromo-5-chloroaniline is a important activity site that can involve multiple substitution and acidylation reaction.

2-bromo-5-chloroaniline can react with fluorophenylboronic acid such as 2,3-difluorophenylboronic acid, 2-fluorophenylboronic acid, 3-fluorophenylboronic acid and 3,5-difluorophenylboronic acid by nucleophilic substitution and produce 4-chloro-2',3'- difluorobiphenyl--2-amine, 4-chloro-2'- fluorobiphenyl-2-amine and so on, which further acetylate producing urea derivatives that can work as fatty-acid binding protein (FABP) inhibitors[8]. 5-chloro-2-(4.4, 5.5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline can be prepared by bis(pinacolato)diboron and 2-bromo-5-chloroaniline by substitution at 2-bromo[9]. Thus, 2-bromo of 2-bromo-5-chloroaniline can involved various substitution reaction for preparing chemical intermediate.
In addition, 1-NH2 and 2-bromo of 2-bromo-5-chloroaniline can involve reaction simultaneously. For example, in Cu-catalyzed Ullman coupling−cyclization, 2-bromo-5-chloroaniline reacted with (S)-cyclopropylglycine and gave the quinoxalinone core, in which 2-bromo involved acidylation reaction, and 2-bromo involved substitution reaction[10]. And the obtained quinoxalinone was reduced to (S)-6-chloro-2-cyclopropyl-1,2,3,4-tetrahydroquinoxaline for further preparing compouds with bromodomain modules.

In conclusion, 2-Bromo-5-chloroaniline have three substituent groups, in which 1- and 2-groups belong to activity sites that can involve multiple reaction.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB6714501.htm.
[2] Yale, H. L., Petigara, R. B. (1976). U.S. Patent No. 3,966,733. Washington, DC: U.S. Patent and Trademark Office.
[3] Yale, H. L., Petigara, R. B. (1977). U.S. Patent No. 4,062,846. Washington, DC: U.S. Patent and Trademark Office.
[4] Katayev, D., Kündig, E. P. (2012). Catalytic Enantioselective Synthesis of a 3-Aryl-3-benzyloxindole (= 3-Aryl-3-benzyl-1, 3-dihydro-2H-indol-2-one) Exhibiting Antitumor Activity. Helvetica Chimica Acta, 95(11), 2287-2295.
[5] Ren, Y., Rousseaux, S. A. (2017). Metal-Free Synthesis of Unsymmetrical Ureas and Carbamates from CO2 and Amines via Isocyanate Intermediates. The Journal of organic chemistry, 83(2), 913-920.
You may like
Lastest Price from 2-BROMO-5-CHLOROANILINE manufacturers
US $0.00/kg2025-05-21
- CAS:
- 823-57-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS
US $200.00-1.00/KG2024-03-25
- CAS:
- 823-57-4
- Min. Order:
- 1KG
- Purity:
- 99%, 99.5% Sublimated
- Supply Ability:
- g-kg-tons, free sample is available