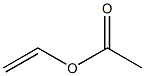
Uses and Toxicity of Poly(vinyl acetate)
Polyvinyl acetate was discovered in Germany in 1912 by Fritz Klatte. The monomer, vinyl acetate, was first produced on an industrial scale by addition of acetic acid to acetylene with a mercury(I) salt[3], but it is now primarily made by palladium catalyzed oxidative addition of acetic acid to ethylene.
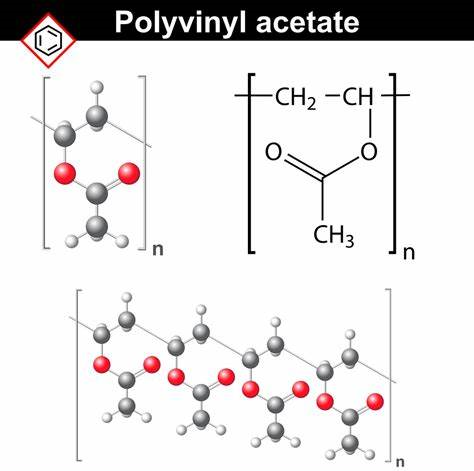
Poly(vinyl acetate) is used as the thermoplastic resin that is hydrolyzed into polyvinyl alcohol in an acid or alkaline solvent and the main raw material to prepare polyvinyl alcohol. When the molecule contains a photosensitizer, it is sensitive to light, and undergoes a decomposition reaction under the action of ultraviolet light or electron beam. It has a positive photosensitive resin property.
Polyvinyl acetate is soluble in a variety of organic solvents and can be copolymerized with a variety of monomers with double bonds to introduce various functional groups with different properties. It is a leathery, colorless thermoplasticmaterial that softens at relatively lowtemperatures and that is relatively stable to lightand oxygen. The polymers are clear and noncrystalline. The chief applications of polyvinylacetate are as adhesives and binders for waterbasedor emulsion paints. Vinyl acetate is conveniently prepared bythe reaction of acetylene with acetic acid.
Rats were orally administered 500 mg/kg for 30 days, and cheese-like changes in their liver and cardiomyocytes were reported. It is safe to be used in food (FDA, §172.615, 2000) as a gum chew and does not enter the human body and is non-toxic. It cannot be absorbed by the body as it is a polymer substance that is insoluble in water and oil.
References
The National Pollutant Inventory. 2005. Acrylamide fact sheet.
International Agency for Research on Cancer. 1994. Summaries and evaluations acrylamide group 2A. Monographs on the evaluation of carcinogenic risks to humans, vol. 60. Lyon, France: International Agency for Research on Cancer.
Agency for Toxic Substances and Disease Registry. 2002. Managing hazardous materials incidents. Vol. III—Medical management guidelines for acute chemical exposures, aniline. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Wetherhold, J. M., Linch, A. L., and Charsha, R. C. 1960. Chemical cyanosis—Causes, effects, and prevention. Archives of Environmental Health 1: 75.
You may like
Related articles And Qustion
See also
Lastest Price from POLY(VINYL ACETATE) manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 9003-20-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $60.00/kg2025-04-15
- CAS:
- 9003-20-7
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000