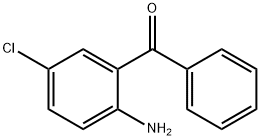
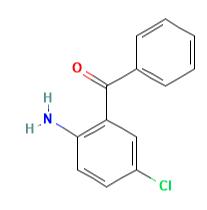
Unveiling the Marvels of 2-Amino-5-chlorobenzophenone
Introduction
2-Amino-5-chlorobenzophenone (ACBP) has emerged as a compound of considerable importance in recent years, attracting attention from researchers across diverse scientific disciplines. Initially recognized for its synthetic utility, It has evolved beyond its origins to find applications in pharmaceuticals, materials science, and beyond. This review delves into the various facets of it, aiming to provide a comprehensive overview of its properties, synthesis methods, biological activities, and industrial applications.
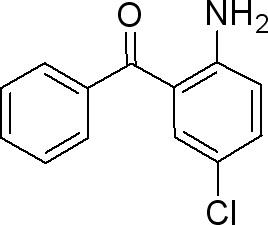
Synthesis
2-Amino-5-chlorobenzophenone can be synthesized through several well-established methods, each varying in complexity and efficiency. One commonly employed route involves the reaction of chloroanilines with benzoyl chlorides under controlled conditions. Alternatively, modifications such as Suzuki-Miyaura coupling or direct amination strategies have been explored to tailor the synthesis toward specific applications. The choice of synthetic pathways often depends on factors such as yield, purity requirements, and scalability, reflecting the versatility of ACBP as a chemical intermediate.
Chemical Properties and Structural Characteristics
At its core, 2-Amino-5-chlorobenzophenone features a benzophenone backbone substituted with an amino and a chloro group. This molecular arrangement imparts distinctive chemical properties, influencing its reactivity and interactions in various environments. It is typically a crystalline solid with a defined melting point and solubility profile, crucial considerations in its handling and application. Structural elucidation techniques such as spectroscopy and X-ray crystallography have provided insights into its three-dimensional arrangement, facilitating a deeper understanding of its behavior at the molecular level.1
Biological Activities and Pharmacological Significance
The biological activities of 2-Amino-5-chlorobenzophenone span a spectrum of pharmacological effects, underpinning its relevance in medicinal chemistry. Studies have highlighted its potential as a scaffold for developing novel pharmaceutical agents, with reported activities ranging from antimicrobial and anticancer properties to enzyme inhibition and neuroprotective effects. Mechanistic investigations into its interaction with biological targets have uncovered pathways that underscore its therapeutic potential, driving ongoing research into optimized derivatives and formulations.2-Amino-5-chlorobenzophenone derivatives have been explored for their potential as:2
Antimicrobial Agents
Certain derivatives exhibit promising antimicrobial activity against a range of pathogens, contributing to efforts in combating infectious diseases.
Anticancer Compounds
Modified versions of it have shown cytotoxic effects on cancer cells in preclinical studies, suggesting potential applications in oncology.
Neurological Disorders
Research indicates that it derivatives may influence neurotransmitter systems, presenting opportunities for novel therapeutic interventions in neurological disorders.
Applications in Materials Science
Beyond its role in pharmacology, ACBP has found applications in materials science, particularly in the synthesis of functional polymers and organic electronic materials. Its ability to undergo facile modifications through organic reactions has facilitated its integration into polymer matrices with tailored physical and electronic properties. ACBP-derived materials have demonstrated utility in fields such as optoelectronics, sensing, and biomedical engineering, leveraging their chemical versatility to enhance device performance and functionality. Beyond pharmaceuticals, 2-Amino-5-chlorobenzophenone finds applications in materials science:3
Photostabilizers
ACBP derivatives are employed as UV absorbers and photostabilizers in plastics, coatings, and polymers. These compounds help mitigate the degradation caused by UV radiation, prolonging the lifespan of materials exposed to sunlight.
Dye Sensitizers
In dye-sensitized solar cells (DSSCs), ACBP derivatives act as sensitizers that absorb light and generate electrons, contributing to the efficiency of solar energy conversion technologies.
Optoelectronics
The electronic properties of ACBP derivatives make them suitable for use in organic light-emitting diodes (OLEDs) and other optoelectronic devices, where they facilitate efficient charge transport and light emission.
Industrial Applications and Future Prospects
In the industrial sector, 2-Amino-5-chlorobenzophenone serves as a valuable precursor in the synthesis of specialty chemicals and fine pharmaceutical intermediates. Its robust synthetic accessibility and diverse chemical reactivity position it favorably for large-scale production processes. Ongoing efforts are focused on expanding its application scope through collaborative research initiatives aimed at exploring new reaction methodologies and optimizing synthetic routes. Looking forward, the integration of ACBP into emerging technologies holds promise for addressing current challenges in healthcare, materials development, and environmental sustainability.4
Conclusion
2-Amino-5-chlorobenzophenone (ACBP) exemplifies a paradigmatic compound whose versatility transcends disciplinary boundaries, encompassing pharmaceuticals, materials science, and industrial chemistry. This comprehensive review has highlighted its synthesis, chemical properties, biological activities, and manifold applications, underscoring its pivotal role in contemporary research and development efforts. As scientific understanding continues to evolve, 2-Amino-5-chlorobenzophenone stands poised to catalyze innovation across diverse sectors, driven by its intrinsic chemical flexibility and multifaceted utility. Future endeavors will undoubtedly unravel new dimensions of ACBP's potential, forging pathways toward transformative advancements in science and technology.
References:
[1] M. GULAM MOHAMED . Growth and characterization of 2-amino-5-chlorobenzophenone (2A-5CB) single crystals[J]. Journal of Crystal Growth, 2007, 300 2: 263-564. DOI:10.1016/j.jcrysgro.2006.11.341.[2] SANDRA CORTEZ-MAYA. Synthesis of 2-Aminobenzophenone Derivatives and Their Anticancer Activity[J]. Synthetic Communications, 2012, 42 1: 46-54. DOI:10.1080/00397911.2010.521435.
[3] RAJESH K. SINGH D. N P Sonia Devi. Synthesis, physicochemical and biological evaluation of 2-amino-5-chlorobenzophenone derivatives as potent skeletal muscle relaxants[J]. Arabian Journal of Chemistry, 2015, 8 3: 285-432. DOI:10.1016/j.arabjc.2011.11.013.
[4] SHEN K. YANG. 2-(Methylamino)-5-chlorobenzophenone Imine: A Novel Product Formed in Base-Catalyzed Hydrolysis of Diazepam[J]. Journal of pharmaceutical sciences, 1996, 85 7: 670-790. DOI:10.1021/js9504245.
You may like
Related articles And Qustion
Lastest Price from 2-Amino-5-chlorobenzophenone manufacturers

US $1.00-4.00/KG2025-05-19
- CAS:
- 719-59-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1KG 100KG 1MT

US $0.00/KG2025-04-21
- CAS:
- 719-59-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month