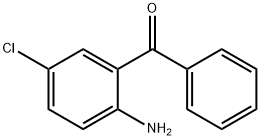
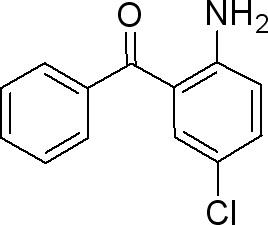
Application of 2-Amino-5-chlorobenzophenone
General description
(2-Amino-5-chlorobenzophenone(CAS No: 719-59-5), 2-Aminobenzophenone derivatives are important compounds in organic chemistry because of their application in heterocyclic synthesis and medicines. 2-Aminobenzophenone has been used as starting material for the synthesis of 1,4-benzodiazepines, proquazone and amfenac as anti-inflammatory agents. It has also been used for the synthesis of Peptidoaminobenzophenones, a novel class of ring open derivatives of 1, 4-benzodiazepines, evaluated for Central Nervous System (CNS) activity. So it was considered to be of interest to synthesize the 2-aminobenzophenones derivatives having all the important pharmacophores required for the CNS activity of benzodiazepines to obtain drugs with high therapeutic index than diazepam. In this 2-amino-5-chlo-robenzophenones are subjected to acetylation by treating with chloroacetylchloride to obtain 2-chloroacetamidobenzophenones which are then reacted with different aniline derivatives in the presence of potassium carbonate and DMF by conventional and microwave irradiation methods to obtain different derivatives.[1]
2-amino-5-chlorobenzophenone crystal was grown using slow evaporation solution growth technique. From FT-IR and FT-RAMAN, the functional and vibrational frequencies of the grown crystals are assigned, that is, N–H stretch at 3320 cm1 and 3318 cm1 and C=O stretch at 1623.7 and 1620 cm1, respectively. The microhardness studies reveal that the hardness of crystal is moderately good. The straight striations, and kinks were observed on the crystal surface during etching.[2]

Figure 1 the molecular formula of 2-Amino-5-chlorobenzophenone
Application and pharmacokinetics
It is also used as antiinflammatory, and antimitiotic agents, p38 MAP kinase inhibitors [4], as well as bradykinin B1 receptor antagonists. 2-Amino-5-chlorobenzophenone derivatives have been used as skeletal muscle relaxants. Benzophenone skeleton has been found in many fungicide including Flumorph®. Benzophenones are also involved in dyes manufacturing and in the field of non-linear optical devices.
2-Aminobenzophenone derivatives were also evaluated as antimitoticagents and a novel class of bradykinin B1 receptor antagonists with excellent receptor occupancy in the CNS of hBK B1 transgenic rats but not a substrate for P-glycoprotein (P-gp) mediated efflux and hence good brain penetration. [3]All these drugs synthesized from 2-aminobenzophenones possess CNS activity. 2-aminobenzo-phenone derivatives were considered to be of our interest because they fulfill all the structure activity requirements as the benzodiazepine contains. According to SAR study rings A and C and substitutions at these rings and the substitution at the amide nitrogen of ring B are important for the activity of benzodiazepines as shown in Fig. 2.

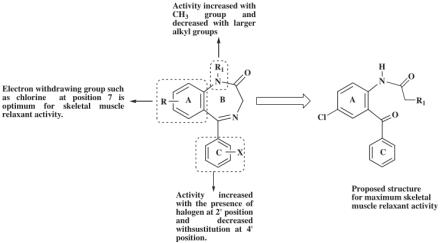
Fig. 2 Important pharmacophore of 1,4-benzodiazepine nucleus.
Mechanism of action
Almost half of the world’s population, especially in the developing countries is affected by malaria. The relatively few drugs available to fight this disease are continuously threatened by the development of resistance. The urgent need for the development of novel agents against malaria which preferable should act through hitherto unexploited mechanisms of action is undisputed. Recently, we described 2,5-diaminobenzophenones carrying acyl or sulfonyl residues at the 5-amino group with significant antimalarial activity. However, compounds also displayed considerable cytotoxicity and therefore unsatisfactory selectivity towards malaria parasites. Best representative in terms of activity and selectivity was the di-isopropyl urea derivative 1 with an IC50of 110 nM and a selectivity index (CC50/IC50(Plasmodium falciparum) of 36. In comparison to the parent non-acylated or non- sulfonylated 5-amino derivative 2 the N-modified compounds were up to 70fold more active . Acylation of the 5-amino group changes two molecular properties: first, the electron-donating 5-substituent is converted into an electron-withdrawing and, second, a more or less spacious lipophilic substituent is added. Often more lipophilic compounds also display enhanced cytotoxicity. We therefore speculated that the replacement of the 5-acylamino moiety could reduce cytotoxicity while possibly antimalarial activity could be preserved. We chose the readily available 2-amino- 5-chlorobenzophenone (3) as the basic scaffold of this series. We extensively varied the acyl residue at the 2-amino group to explore the influence of this moiety on antimalarial activity as well as theselectivity towards the parasites.

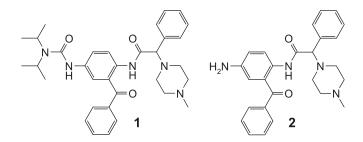
Figure 3 di-isopropyl urea derivative IC50¼ 110 nM, 2 5-amino derivative IC50¼ 6.5 mM.
Previously a series of 5-acylaminobenzophenones with considerable antimalarial activity. Unfortunately, most compounds also displayed high cytotoxicity resulting in low selectivity towards malaria parasites. Through the replacement of the 5-acylamino moiety by simple chlorine and further modifications of the 2-acylamino residue that could obtain inhibitors with improved selectivity towards malaria parasites combined with an acceptable reduction of antimalarial activity. a series of 5-acylamino-benzophenones with considerable antimalarial activity. There value was however reduced by high cytotoxicity and therefore low selectivity of most compounds. This speculated that it might possible to separate antimalarial from unwanted cytotoxic activity. Through replacement of the 5-acylamino moiety by a simple chlorine and modification or the 2-acylamino residue we obtained inhibitors which were slightly less active against cultured malaria parasites but showed improved selectivity against the parasites. The selectivity index could be improved 4fold from 36 to 150. [3]
Synthesis
2-aminobenzophenone is used as starting material in the synthesis of 1,4-benzodiazepines.
General procedure for the synthesis1-33:2-Amino-5-chlorobenzophenone Schiff bases1-33 were synthesized by irradiating a pulse of MW at 100 W maintaining a temperature for a span of 1 min at 100 ℃ to the reaction mixture of 2-amino-5-chlorobenzophenone (1 mmol), appropriate aldehyde (1 mmol), acetic acid (2-drops), and ethanol (5 ml). Completion of reaction was monitored by thin layer chromatography. The reaction mixture was left at room temperature until precipitates appeared. Solid mass was washed with hexane to afford pure product in quantitative yields.
The syntheses of 2-amino-5-chlorobenzophenone and 2-(chloroacetamido)-5-chlorobenzophenone were carried out by literature methods (Sternbach et al., 1961, 1962). In this method, 2-amino-5-chlorobenzophenone (1) (1 mole) and chloroacetylchloride (2 mole) in toluene was refluxed for 2.5 h to expel most of the formed hydrogen chloride. The solution was then cooled, washed with ice cold dilute aqueous ammonia solution, dried with anhydrous sodium sulfate, filtered and concentrated in vacuo. The recrystallization of the crude residue from alcohol afforded 82% of 2-(chloroacetamido)-5-chlorobenzophenone (2); mp 119–122 °C, lit.119–121 °C.[4]

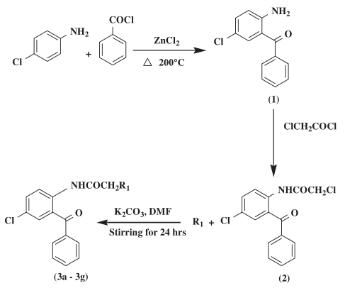
Figure 4 Synthesis of 2-(chloroacetamido)-5-chlorobenzophenone
References
1.Arshia, Khan A. K. & Khan K. M. et al., "Antibiofilm potential of synthetic 2-amino-5-chlorobenzophenone Schiff bases and its confirmation through fluorescence microscopy," Microbial pathogenesis, Vol.110(2017), pp.497-506.
3.Heinrich S., Altenkämper M. & Bechem B. et al., "2-Acylamino-5-chlorobenzophenones with enhanced selectivity towards malaria parasites," European Journal of Medicinal Chemistry, Vol.46, No.4(2011), pp.1331-1342.
Related articles And Qustion
Lastest Price from 2-Amino-5-chlorobenzophenone manufacturers

US $1.00-4.00/KG2025-05-19
- CAS:
- 719-59-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1KG 100KG 1MT

US $0.00/KG2025-04-21
- CAS:
- 719-59-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month