Unveiling (R)-DIFLUORPHOS(TM): A Catalyst for Change in Organophosphorus Chemistry
Introduction
The world of organophosphorus chemistry is continually evolving, with new catalysts frequently emerging to enhance synthetic efficiencies and offer novel pathways for chemical reactions. Among these innovations, (R)-DIFLUORPHOS(TM) stands out as a significant advancement. This specialized ligand is gaining traction in academic and industrial settings due to its unique properties and versatile applications. Its distinct molecular structure and high reactivity make it particularly useful in enantioselective synthesis, appealing to researchers and manufacturers alike who seek precision and efficiency in their chemical processes[1].

Figure 1 Characteristics of (R)-DIFLUORPHOS(TM)
Synthesis Method
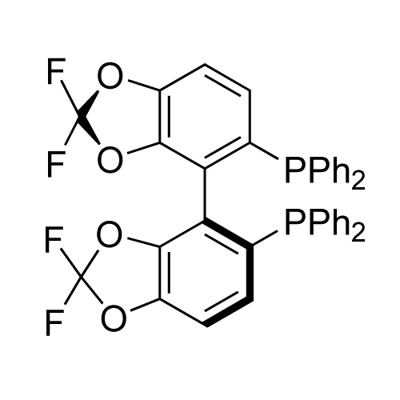
(R)-DIFLUORPHOS(TM), chemically known as Bis[(R)-(+)-2,2-difluoro-1,1-biphenyl-2-yl]phosphine, is synthesized through a meticulous process that involves the chiral resolution of biphenyl derivatives followed by a complexation with a suitable phosphine source. The synthesis begins with the diastereoselective fluorination of biphenyl compounds, which is crucial for achieving the desired optical purity. Subsequent phosphination steps are carefully controlled to ensure the formation of the phosphine moiety without compromising the ligand’s stereochemical integrity. The result is a highly pure and optically active phosphine ligand, tailored for high-performance catalytic applications.
Main Components
The primary component of (R)-DIFLUORPHOS(TM) is its unique biphenyl structure, which features two phenyl rings connected at a single point, each bearing fluorine atoms at the ortho positions. This structural arrangement is critical as it imparts the ligand with remarkable stability and rigidity, enhancing its efficacy in catalytic cycles. The difluoro groups not only increase the electron-withdrawing properties of the ligand but also enhance its lipophilicity, which is beneficial for various organic transformations. Additionally, the specific arrangement of the fluorine atoms adds to the steric bulk around the phosphorus center, further influencing the ligand's selectivity and reactivity in asymmetric synthesis. This contributes to more controlled and predictable outcomes in complex reaction environments.
Applications
(R)-DIFLUORPHOS(TM) has found extensive use in asymmetric catalysis, particularly in transformations that require high enantioselectivity. One of the most notable applications is in the asymmetric hydrogenation of ketones, a fundamental reaction in the production of pharmaceuticals and fine chemicals. The ligand’s ability to induce chirality in substrates that are typically challenging to enantioselectively reduce underscores its utility in synthetic organic chemistry. Moreover, (R)-DIFLUORPHOS(TM) is also employed in cross-coupling reactions where its properties facilitate the formation of carbon-carbon bonds with excellent stereoselectivity. Additionally, its use extends to the synthesis of natural products and agrochemicals, where precise stereocontrol is crucial. The ligand’s robust performance in catalyzing arylation and alkylation reactions further broadens its applicability across various chemical sectors, enhancing the synthesis of complex molecules in a more environmentally friendly and cost-effective manner. This versatility not only highlights the ligand’s pivotal role in modern chemistry but also points to its potential in future pharmaceutical and material science innovations[2].
Storage Methods
Proper storage of (R)-DIFLUORPHOS(TM) is essential to maintain its effectiveness and longevity. The ligand should be stored in a cool, dry place, away from direct sunlight and moisture. It is typically provided in sealed containers under an inert atmosphere, usually nitrogen, to prevent oxidation and degradation. Handling should always be conducted in an environment free from oxygen and moisture, such as a glovebox or under dry nitrogen flow, to preserve the ligand’s reactivity and prevent any possible hydrolysis or decomposition.
Conclusion
As the chemical industry continues to advance towards more sustainable and efficient practices, catalysts like (R)-DIFLUORPHOS(TM) play a pivotal role. With its unique structural features and impressive catalytic capabilities, (R)-DIFLUORPHOS(TM) is set to remain a key player in the field of organophosphorus chemistry. Its development not only represents a significant scientific achievement but also highlights the ongoing innovation within the chemical industry, aimed at overcoming some of the most challenging barriers in synthetic chemistry. As researchers continue to explore and expand the applications of this versatile ligand, the potential for discoveries and advancements appears limitless.
References
[1]Prevost S, Ayad T, Genet J P, et al. DIFLUORPHOS and SYNPHOS in asymmetric catalysis: Synthetic applications[J]. Journal of Chemical Sciences, 2014, 126: 325-340.
[2]Ge S, Hartwig J F. Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions[J]. Journal of the American Chemical Society, 2011, 133(41): 16330-16333.
Related articles And Qustion

US $0.00-0.00/KG2025-07-29
- CAS:
- 503538-70-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000

US $2.00-5.00/KG2025-07-09
- CAS:
- 503538-70-3
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons