Triphenyl phosphite: synthesis, applications in organic synthesis and safety
General Description
Triphenyl phosphite is a significant trivalent phosphorus compound that has versatile applications in organic synthesis. Its synthesis has been optimized using deprotection conditions, which have also been successfully applied to the deprotection of a phosphite-borane for the first time. Triphenyl phosphite acts as a reactant in the synthesis of novel diruthenium di-μ-hydrido complexes and as a catalyst in the highly selective allylic alkylation with a carbon nucleophile at the more substituted allylic terminus catalyzed by iridium complexes. However, it has potential safety hazards, and it is essential to handle it with care, use appropriate protective equipment, and follow recommended safety protocols to avoid any potential harm to people or the environment.

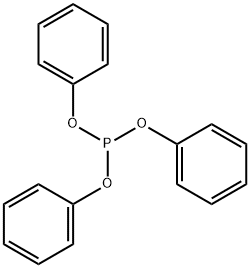
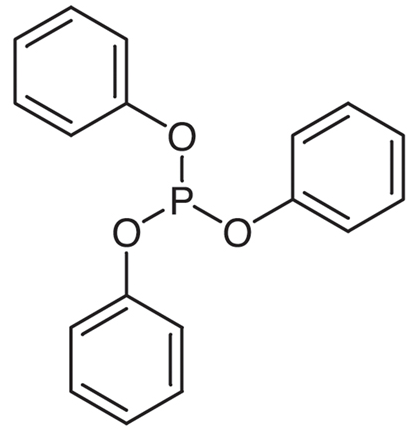
Figure 1. Triphenyl phosphite
Synthesis
Triphenyl phosphite is an important trivalent phosphorus compound and its synthesis has been optimized using deprotection conditions. The protection of trivalent phosphorus compounds with complexation with borane can be easily removed by reaction with Merrifield resin-supported piperazine and 1-methylpiperazine. These deprotection conditions have been optimized for different types of phosphorus compounds, and the resulting solutions can be used without any intermediate work-up or purification process. The deprotection of phosphine-boranes with mol. sieves 4 Å in a mixture of an ethereal solvent and an alc. provided deprotected free phosphines in quant. yields. The phosphines can be obtained by a simple filtration/crystallization procedure in most cases. Notably, this method has been successfully applied to the deprotection of a phosphite-borane for the first time. This optimized synthesis of Triphenyl phosphite provides a useful tool for the preparation of various organic phosphorus compounds. 1
Applications in organic synthesis
Reactant
Triphenyl phosphite has versatile applications as a reactant, particularly in the synthesis of novel diruthenium di-μ-hydrido complexes. These complexes, denoted as [CpRu(PR3)(μ-H)2RuCp] (2a-g), were synthesized by reacting [{Cp*Ru}2(μ-H)4] with a phosphorus ligand lacking aryl substituents. The complexes exhibit fluxional intramolecular migration of the phosphorus ligand between the two ruthenium centers. This phenomenon is supported by variable temperature NMR spectroscopic studies and preliminary DFT calculations. Crystal structure determination of 2a-g reveals that the Ru-Ru-P angle decreases as the electronegativity of the phosphorus ligand increases. The activation enthalpy for the migration process decreases in the same order. The migration process occurs within the molecule, as indicated by low activation entropy values and the absence of exchange with added phosphines. The observations suggest the presence of through-space back-bonding interaction between ruthenium and phosphorus atoms. These findings highlight Triphenyl phosphite's role as a key reactant in the synthesis and study of complex organometallic compounds with intriguing intramolecular dynamics. 2
Catalyst
Triphenyl phosphite demonstrates significant applications as a catalyst, particularly in the highly selective allylic alkylation with a carbon nucleophile at the more substituted allylic terminus catalyzed by iridium complexes. For instance, [Ir(cod)Cl]2/P(OPh)3 catalyzed the reaction of allylic compounds, resulting in alkylated products with high selectivity. The reaction, catalyzed by this system, effectively constructs quaternary carbon centers, which are valuable structural motifs in organic synthesis. The reaction of specific allylic compounds with carbon nucleophiles yielded the desired alkylated products with high efficiency and selectivity. For example, the reaction of certain allylic compounds with a carbon nucleophile resulted in alkylated products with yields as high as 89%. Additionally, the reaction of other allylic compounds with a different carbon nucleophile produced alkylated products with a yield of 85%. These results underscore the utility of triphenyl phosphite as a catalyst in facilitating highly selective allylic alkylation reactions, enabling efficient construction of quaternary carbon centers in organic molecules. 3
Safety
Triphenyl phosphite has potential safety hazards. It reacts with water and is an irritant to the skin, eyes, and respiratory tract. It may cause skin sensitization, and high concentrations may cause nervous system effects. Emergency treatment involves TOCP and related agents, which can cause acute gastrointestinal symptoms like vomiting, diarrhea, and abdominal pain. Patients may also develop peripheral neuropathy or flaccid paralysis, which can resolve in some cases over two to three months but may be permanent in others. Additionally, the compound is toxic to aquatic organisms. Therefore, it is essential to handle Triphenyl phosphite with care, use appropriate protective equipment, and follow the recommended safety protocols to avoid any potential harm to people or the environment. 4
Reference
1. A novel mild deprotection method for phosphine-boranes. Bulletin of the Chemical Society of Japan, 2004, 77(10): 1931-1932.
2. Ohki Y, Suzuki H. Migration of a phosphane ligand between the two metal centers in diruthenium hydrido complexes. Angew Chem Int Ed Engl, 2002, 41(16): 2994-2997.
3. Takeuchi R, Kashio M. Highly selective allylic alkylation with a carbon nucleophile at the more substituted allylic terminus catalyzed by an iridium complex: an efficient method for constructing quaternary carbon centers. Angew Chem Int Ed Engl, 1997, 36(3): 263-265.
4. Triphenyl phosphite. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases, 7630.
Related articles And Qustion
See also
Lastest Price from Triphenyl phosphite manufacturers

US $700.00/kg2025-08-18
- CAS:
- 101-02-0
- Min. Order:
- 920kg
- Purity:
- 98.0%
- Supply Ability:
- 36.8 ton

US $10.00/kg2025-04-21
- CAS:
- 101-02-0
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON