Triphenyl phosphite: properties and applications
General Description
Triphenyl phosphite is a colorless to pale yellow liquid with a mild aromatic odor. It is soluble in organic solvents and consists of a phosphorus atom bonded to three phenyl groups. It reacts with aldehydes and ketones to form phosphonates. It possesses unique characteristics and finds wide-ranging applications. Triphenyl phosphite is an organic phosphorus compound with unique characteristics and diverse applications. It plays a crucial role in organic synthesis, polymer preparation, and metal coordination chemistry, offering enhanced efficiency and selectivity in numerous chemical processes.

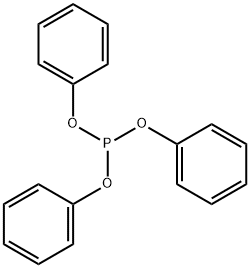
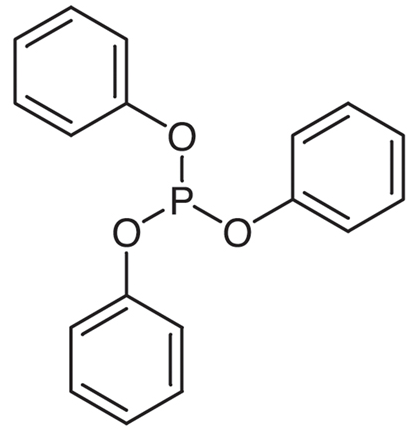
Figure 1. Triphenyl phosphite
Properties
Triphenyl phosphite (C18H15OP) is a chemical compound that appears as a colorless to pale yellow liquid with a mild aromatic odor. It has a molecular weight of 310.28 g/mol, a melting point of 27-29°C, and a boiling point of 360-361°C. Triphenyl phosphite is soluble in organic solvents such as ethanol, benzene, and chloroform, with a density of 1.246 g/cm3 at 25°C. It consists of a phosphorus atom bonded to three phenyl groups. It is stable at room temperature and does not readily react with air. Triphenyl phosphite has good solubility and can dissolve in most organic solvents. This compound is stable under normal conditions but can degrade in the presence of strong acids or oxidizing agents. It exhibits reactivity by reacting with aldehydes and ketones to form corresponding phosphonates. 1
Application
Catalytic role in organic synthesis
Triphenyl phosphite has been utilized as a catalyst in the intermolecular addition reactions of sulfonamides to alkenes and conjugated dienes. When combined with low loading of (triphenyl phosphite)gold(I) chloride and silver triflate, Triphenyl phosphite exerts catalytic activity in these reactions. Under conventional thermal conditions or microwave irradiation, the catalytic mixture promotes the addition reaction of sulfonamides to alkenes, resulting in regioselective hydroamination at the internal carbon atom of terminal alkenes. Additionally, the reaction can be carried out at room temperature for dienes, wherein the less substituted double bond of the diene undergoes regioselective hydroamination. The use of triphenyl phosphite as a catalytic agent provides an effective means to achieve intermolecular addition reactions between sulfonamides, alkenes, and conjugated dienes, allowing for the selective formation of desired products. 2
Metal coordination
Triphenyl phosphite also serves as a precursor for metal coordination compounds. Various organometallic complexes can be obtained by reacting it with transition metal salts. These complexes hold immense significance in organic synthesis, catalysis, and materials science. Triphenyl phosphite has been extensively studied for its role as a ligand in the coordination chemistry of metal complexes. In particular, Triphenyl phosphite forms solvate complexes with copper(I) ions, exhibiting various coordination geometries and properties. When used as a ligand for copper(I) ions, Triphenyl phosphite forms tetrahedral solvate complexes both in solution and solid state. These complexes have been characterized using techniques such as Extended X-ray Absorption Fine Structure spectroscopy and Copper-63 (^63Cu) and Copper-65 (^65Cu) Nuclear Magnetic Resonance spectroscopy. Overall, Triphenyl phosphite demonstrates its utility as a ligand in copper(I) solvate complexes, playing a crucial role in determining their coordination geometries and providing valuable insights into the structural properties of these systems. 3
Polymer preparation
Triphenyl phosphite plays a crucial role in the synthesis of novel polyamides (PAs) through the use of molten tetrabutylammonium bromide. In a study, a monomer diacid was synthesized in four steps. This diacid monomer featured an anthracenic group and an amino acid S-valine pendant group. The synthesized PAs demonstrated excellent thermal stability, withstanding temperatures up to 335°C before experiencing a weight loss of 10% in a nitrogen atmosphere. Additionally, their glass transition temperatures ranged from 177 to 185°C. Overall, the use of Triphenyl phosphite in conjunction with TBAB presented an efficient and environmentally friendly method for the synthesis of novel aromatic polyamide. 4
Reference
1. National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
2. Giner X, Nájera C. (Triphenyl phosphite)gold(I)-catalyzed intermolecular hydroamination of alkenes and 1,3-dienes. Org Lett, 2008, 10(14):2919-2922.
3. Nilsson KB, Persson I. The coordination chemistry of copper(I) in liquid ammonia, trialkyl and triphenyl phosphite, and tri-n-butylphosphine solution. Dalton Trans, 2004, (9):1312-1319.
4. Mallakpour S, Mirkarimi F. Synthesis and characterization of novel, optically active polyamides derived from S-valine natural amino acid and bulky anthracenic side chain. Amino Acids, 2010, 39(5):1255-1263.
Related articles And Qustion
Lastest Price from Triphenyl phosphite manufacturers

US $700.00/kg2025-08-18
- CAS:
- 101-02-0
- Min. Order:
- 920kg
- Purity:
- 98.0%
- Supply Ability:
- 36.8 ton

US $10.00/kg2025-04-21
- CAS:
- 101-02-0
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON