Trifluoromethyl Iodide: A Potent Chemical Compound Revolutionizing the Field of Chemistry
Introduction
Trifluoromethyl iodide, commonly referred to as CF3I, has emerged as a standout chemical compound within the scientific arena, captivating the interest of researchers, notably in the domain of organic chemistry. With its distinctive molecular architecture and adaptable characteristics, CF3I has firmly established itself as a pivotal resource in an array of chemical processes and practical applications. Its significance transcends conventional boundaries, driving exploration and innovation across diverse scientific disciplines[1].
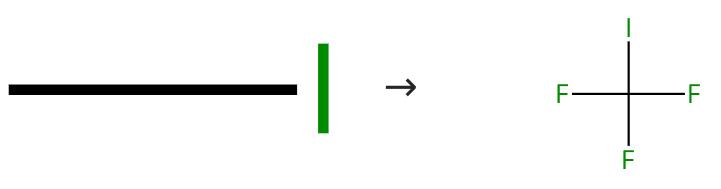
Figure 1: Synthesis pathway of Trifluoromethyl iodide
Synthesis
The synthesis of trifluoromethyl iodide presents a fascinating array of methodologies, showcasing its versatility in chemical production. Among the prominent routes, the reaction between iodine and trifluoroacetic acid emerges as a widely utilized method, renowned for its efficacy in yielding high-purity CF3I under meticulously controlled conditions. Furthermore, alternative pathways include the reaction of iodine with trifluoromethyl sulfide or the direct fluorination of iodomethane, each offering unique advantages and considerations in the synthesis process. These diverse approaches underscore the adaptability of CF3I synthesis, catering to the diverse needs of researchers and industries alike.
Composition
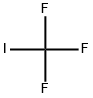
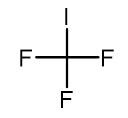
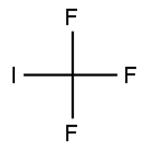
Trifluoromethyl iodide, with its molecular formula CF3I, embodies a precise amalgamation of one carbon atom, three fluorine atoms, and a single iodine atom. This cohesive arrangement forms a tetrahedral molecular geometry, endowing CF3I with unparalleled stability and reactivity essential for driving diverse chemical transformations and innovations.
At its core, trifluoromethyl iodide comprises a single iodine atom intricately bonded to three fluorine atoms and one carbon atom, encapsulating the molecular formula CF3I. Exhibiting a tetrahedral molecular geometry, this distinctive arrangement endows CF3I with both remarkable stability and reactivity, rendering it indispensable in an array of chemical endeavors.
Main Components
Trifluoromethyl iodide's fundamental constituents encompass iodine (I) and fluorine (F), with carbon (C) occupying a pivotal role as the central atom within the molecule. The incorporation of fluorine atoms endows CF3I with unparalleled electronegativity and chemical stability, establishing it as a premier reagent in the realm of organic synthesis. This unique amalgamation of elements underscores CF3I's indispensability in driving forward transformative advancements in the field of chemistry.
Uses
Trifluoromethyl iodide stands as a versatile cornerstone in various fields, including organic synthesis, pharmaceuticals, agrochemicals, and materials science. Its role as a precursor in synthesizing fluorinated compounds is pivotal, particularly in the pharmaceutical sector where these compounds are instrumental in developing drugs with superior pharmacokinetic profiles, enhancing drug efficacy and safety. Furthermore, CF3I's capability to introduce fluorine atoms into organic molecules lends itself to the production of specialty chemicals like pesticides and herbicides, augmenting their biological activity and environmental sustainability. Additionally, in materials science, trifluoromethyl iodide plays a crucial role in crafting fluorinated polymers renowned for their exceptional thermal stability and chemical resistance, facilitating the creation of durable and versatile materials for various industrial applications. The multifaceted utility of trifluoromethyl iodide underscores its indispensability in driving innovation and advancement across a myriad of scientific disciplines.
Storage
Proper storage of trifluoromethyl iodide is essential to ensure its stability and safety. It should be stored in a cool, dry place away from direct sunlight and sources of heat or ignition. Additionally, CF3I should be kept in tightly sealed containers to prevent exposure to moisture and air, which can degrade its quality over time. Careful handling and adherence to safety protocols are crucial when working with trifluoromethyl iodide due to its reactive nature and potential hazards.
Conclusion
In conclusion, trifluoromethyl iodide stands as a pivotal chemical compound that has revolutionized the field of chemistry with its diverse applications and reactivity. From its synthesis to its utilization in organic synthesis, pharmaceuticals, and materials science, CF3I continues to drive innovation and advancements in various scientific disciplines. As researchers continue to explore its potential, trifluoromethyl iodide remains at the forefront of chemical innovation, offering promising opportunities for the development of novel compounds and materials[2].
References
[1]Takechi N, A??t-Mohand S, Médebielle M, et al. Nucleophilic trifluoromethylation of acyl chlorides using the trifluoromethyl iodide/TDAE reagent[J]. Tetrahedron Letters, 2002, 43(24): 4317-4319.
[2]A?t-Mohand S, Takechi N, Médebielle M, et al. Nucleophilic trifluoromethylation using trifluoromethyl iodide. A new and simple alternative for the trifluoromethylation of aldehydes and ketones[J]. Organic Letters, 2001, 3(26): 4271-4273.
You may like
Related articles And Qustion
See also
Lastest Price from Trifluoromethyl iodide manufacturers
US $0.00/KG2025-11-21
- CAS:
- 2314-97-8
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons
US $0.00/kg2025-04-04
- CAS:
- 2314-97-8
- Min. Order:
- 25kg
- Purity:
- 99%
- Supply Ability:
- 1ton