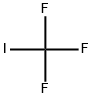
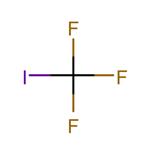
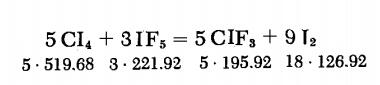Uses
Trifluoroiodomethane is used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires. It is also an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals. It is involved in the rhodium-catalyzed alfa-trifluoromethylation of alfa,beta-unsaturatedketones. It plays an important role as catalyst in the enantioselective alfa -trifluoromethylation of aldehydes through photoredox organocatalysis using a readily available iridium photocatalyst.
Reactions
Trifluoromethyl iodide reacts with [AuMeL] to give [AuMe2(CF3)L] and [AuIL](L = PMe3 or PMe2Ph), or [Au(CF3)L] and Mel (L = PPh3), or a mixture of these products (L = PMePh2). In some cases reaction of [AuMe(PMe3)] with CF3I gives [AuMe(CF3)I(PMe3)]. Evidence is presented that the reactions proceed, at least in part, by a free-radical chain mechanism.
Chemical Properties
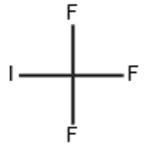
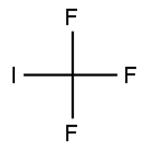
Trifluoromethyl iodide is a colourless gas, it is stable under normal temperatures and pressure. However, upon decomposition, it may form acid halides such as hydrogen fluoride (HF) and hydrogen iodide. It is incompatible with oxidizing materials.
Uses
Reagent used in the rhodium-catalyzed α-trifluoromethylation of α,-unsaturated ketones.
Preparation
Trifluoromethyl iodide's synthesis method:A glass flask provided with a gas outlet is filled with 80 g. (0.153 mole) of CI4 and 30 g. (0.135 mole) of IF5. The gas outlet is connected via short rubber tubes to several gas traps cooled with liquid nitrogen. Agitation of the vessel produces vigorous evolution of gas. When the reaction subsides, the system is heated for 30 min at 90-100°C. The condensate in the gas traps is then washed with 5% NaOH and fractionated. The yield is 90%.
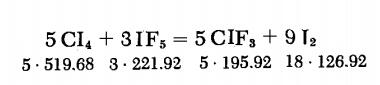
Synthesis Reference(s)
Journal of the American Chemical Society, 107, p. 5014, 1985
DOI: 10.1021/ja00303a042
Flammability and Explosibility
Not classified