ChemicalBook > Articles Catagory List >Chemical-Reagents >triethoxy-1h-1h-2h-2h-perfluorodecylsilane-application
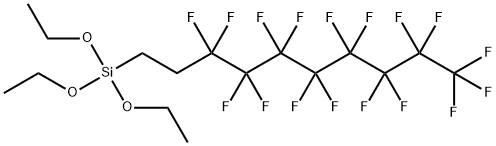
Triethoxy-1H,1H,2H,2H-perfluorodecylsilane--Application
Feb 17,2020
1H,1H,2H,2H-Perfluorodecyltriethoxysilane, also named perfluoroalkylethyltrimethoxysilane (PFATES), is used to bond poly(tetrafluoroethylene) films to silicon wafers and it is also utilized in anti-reflective coating, release coating and soil repellent coating because of its special chemical structure.
Aliphatic, fluorinated aliphatic or substituted aromatic hydrocarbon substituents are the hydrophobic entities which enable silanes to induce surface hydrophobicity and the organic substitution of the silane must be non-polar [1]. How does a silane modify a surface? Most of the widely used organosilanes have one organic substituent and three hydrolyzable substituents. In the vast majority of surface treatment applications, the alkoxy groups of the trialkoxysilanes are hydrolyzed to form silanol-containing species. Reaction of these silanes involves four steps. Initially, hydrolysis of the three labile groups occurs. Condensation to oligomers follows. The oligomers then hydrogen bond with OH groups of the substrate. Finally, during drying or curing, a covalent linkage is formed with the substrate with concomitant loss of water. Although described sequentially, these reactions can occur simultaneously after the initial hydrolysis step. At the interface, there is usually only one bond from each silicon of the organosilane to the substrate surface.
Hydrophobic and Hydrophilic are frequently used descriptors of surfaces (scheme 1). A surface is hydrophobic if it tends not to adsorb water or be wetted by water, and a surface is hydrophilic if it tends to adsorb water or be wetted by water. Silanes can be used to modify the interaction of boundary layers of solids with water with a high degree of control, effecting variable degrees of hydrophobicity or hydrophilicity [2].
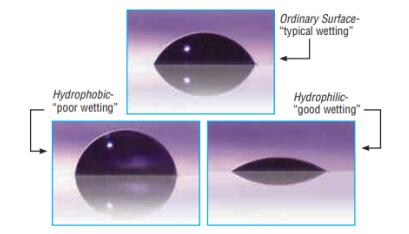
Scheme1 Hydrophobic and Hydrophilic Surface
PPG company developed the autophobic water repellent surface after being treated with PFATES. The developed surface has high water repellency and high lubricity and the durable water and dirt repellency of a substrate surface are provided by applying to the substrate surface a composition comprising PFATES compound and an aprotic solvent which renders the surface treatment autophobic, and as the substrate surface becomes treated with the PFATES, the composition is repelled by the treated substrate surface [3].
Similarly, the same inventor developed a substrate surface with high water repellancy and high lubricity. Durable water and dirt repellency of a substrate surface are provided by applying to the substrate surface a select combination of fluorinated compounds and the high water repellency is provided by perfluoroalkyl silanes which bond to the substrate surface, while high lubricity is provided by the addition of fluorinated olefin telomers [4].
Reference
[1] https://www.gelest.com/product/SIH5841.2/
[2] Barry Arkles, Hydrophobicity, Hydrophilicity and Silane Surface Modification, Gelest, Inc., Version 2
[3] George B. Goodwin, Autophobic water repellent surface treatment, EP0745568B1
[4] Helmut Franz and George B. Goodwin, Water repellent surface treatment, US5308705A
Related articles And Qustion
Lastest Price from 1H,1H,2H,2H-Perfluorodecyltriethoxysilane manufacturers
1H,1H,2H,2H-Perfluorodecyltriethoxysilane

US $0.00-0.00/kg2025-06-13
- CAS:
- 101947-16-4
- Min. Order:
- 0.0001kg
- Purity:
- 99%
- Supply Ability:
- 200000kg
1H,1H,2H,2H-Perfluorodecyltriethoxysilane

US $1.00/kg2025-06-13
- CAS:
- 101947-16-4
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 97%min
- Supply Ability:
- 2mt per month