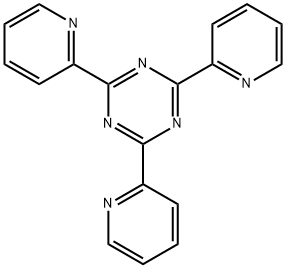
TPTZ, a color former for quantitative determination of iron
2,4,6-Tripyridyl-S-triazine (TPTZ), with the molecule formula of C18H12N6 [1] is the most widely used analytical reagent for the spectrophotometric determination of phenols, phonetic drugs which easily undergoes exudation under experimental conditions. It is widely used for the determination of Iron [2].

Mechanism of Action
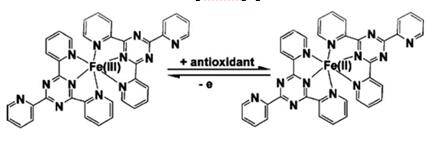
Under the proposed experimental conditions, the FeCl3 oxidised the drug and itself converted to ferrous [Fe+2] which forms a complex with TPTZ to produce a colour solution with a λmax of 595 nm (blue to violet colour).The drug solution is mixed with the FeCl3 and then the oxidised to form of drug. The Fe+2 forms a violet colour complex with the TPTZ solution. Here ferric chloride acts as the strong oxidising agent [2].
Introduction to Procedures
Here, we introduced a method of quantitative determination for iron with TPTZ. In this method of quantitative determination for iron contained in a sample water, first, a dithionite, such as an anhydrous salt of sodium dithionite, is added to the sample water. By this addition, colloidal and sedimentary iron contents contained in the sample water are ionized and dissolved in water, and further trivalent iron ions contained in the sample water are reduced to divalent iron ions. Subsequently, a color former capable of reacting with divalent iron ions to thereby attain a color development is added to the sample water having the dithionite added thereto, and the quantitative determination of iron in the sample water is carried out according to absorption photometry. TPTZ can be used as such a color former.
A quantitative analysis step of iron, that is, divalent iron is performed on the sample water. The quantitative analysis process is performed by absorptiometry. Here, first, TPTZ, a predetermined color former is added to the sample water (The color former used here is not particularly limited as long as it develops color by reacting with divalent iron ions). Usually, the color former is preferably added to the sample water in the form of an aqueous solution or a water-soluble organic solvent solution such as an alcohol solution.
When TPTZ is used as the color former, it is preferable to add ethanol to the sample water before adding TPTZ. If the sample water is water containing sodium polyacrylate as a dispersant to prevent scale formation, such as boiler water, suspended matter is generated when TPTZ is added, and this suspended matter is subjected to spectrophotometry. This suspended substance has a color development wavelength of TPTZ. Since there is a tendency to increase the absorbance, the amount of iron in the sample water determined based on this absorbance may become larger than the actual amount of iron. This is particularly noticeable when TPTZ is added to sample water with a pH of 4.2 or lower. When ethanol is added to the sample water, the formation of such suspended matters is suppressed, and the absorption spectrophotometry can be carried out stably. In general, the amount of ethanol added is preferably 2 to 5 milliliters with respect to 10 milliliters of sample water.
Next, for the sample water to which the color former is added, the absorbance of the color developed by the color former is measured, and the amount of divalent iron ions contained in the sample water is determined from the measurement result. Based on this, the amount of divalent iron ions contained in the sample water is determined. At this time, in order to reduce the measurement error due to the turbidity of the sample water, it is preferable to correct the absorbance at the coloring wavelength by measuring a blank of the sample water in advance and using this measurement value [3].
Reference
[1] https://pubchem.ncbi.nlm.nih.gov/compound/2_4_6-Tripyridyl-s-triazine
[2] https://learning.oreilly.com/library/view/pharmaceutical-analysis/9789332515659/xhtml/chapter048.xhtml
[3] https://patents.glgoo.top/patent/WO2007105322A1/en?q=TPTZ
See also
Lastest Price from TPTZ manufacturers

US $2.20-8.80/kg2025-09-08
- CAS:
- 3682-35-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $0.00/Kg/Bag2025-04-21
- CAS:
- 3682-35-7
- Min. Order:
- 1KG
- Purity:
- 99%min HPLC
- Supply Ability:
- 100KGS