Toxity of Heptane
One of the most versatile chemicals for industrial applications — ranging from anesthetics to solvents — is heptane. As a powerful industrial chemical, it takes a high level of skill and chemical handling know-how to ensure that it is done safely.
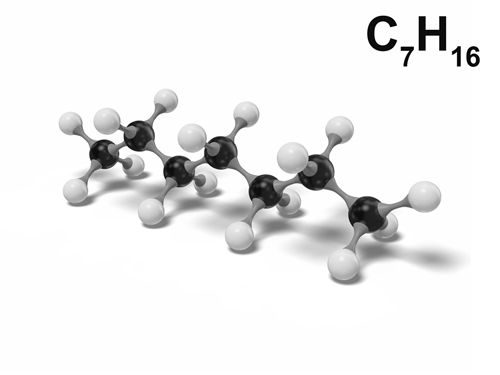
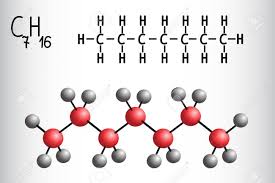
Heptane is a chemical derived from petroleum and a member of the alkane series. It is classified as a “straight-chain” alkane. As a chemical, it is a colorless liquid hydrocarbon. Heptane is considered the standard for octane ratings, such as those listed on gasoline pumps in the United States and around the world. It is also highly flammable and considered to be one of the more useful chemicals of its kind for industrial purposes. Because it is a liquid, heptane is much easier to transport than many other chemicals.
Industrial Applications
Heptane is consistently ranked among the most useful industrial chemicals because of its ideal chemical properties. Among many other applications, heptane is used for anesthetics, cements, compounders, inks, lab reagents, organic synthesis and solvents. In addition to industrial use, heptane is also widely used in science laboratories as a solvent.
Toxity of Heptane
Although some chemical-specific risk data are available for the ingestion route of exposure to nheptane, these data are quite limited and carry a large quantitative uncertainty leading to a larger than acceptable overall uncertainty factor adjustment to support a data-based risk assessment. The generic criterion for non-carcinogens is protective relative to the available (but inadequate) toxicological data.
The Department considered ingestion as well as inhalation exposure under a screening-level showering scenario at 100 ppb n-heptane in water. On the basis of this analysis, inhalation showering exposure is predicted to be more than two orders of magnitude below occupational exposure limits and the odor threshold for n-heptane.
Pharmacokinetic studies have been summarized by the U.S.EPA (1989). n-Heptane appears to share narcotic, central nervous system depressing properties with other volatile alkanes. These effects have been reported in acute inhalation studies, and to a lesser extent, during the course of somewhat longer duration exposures (Biodynamics Inc., 1980).
It is not clear whether decreased auditory sensitivity observed by Simonsen and Lund (1995) is a central nervous system effect. No central nervous system-related effects were observed with either ingestion or intraperitoneal exposure. Most studies of n-heptane toxicology have focused on inhalation exposure since occupational inhalation exposure to n-heptane vapor released from its use as a solvent is the most common exposure.
It is necessary to consider whether such studies can be useful in assessing the ingestion toxicity of n-heptane. In general, inhaled gases that are systemically absorbed from the lungs into blood are transported to the brain before returning to the heart from where the enter the circulatory system as a whole.
Related articles And Qustion
See also
Lastest Price from Heptane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 142-82-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 142-82-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons