Heptane: Application, preparation and toxicity
General description
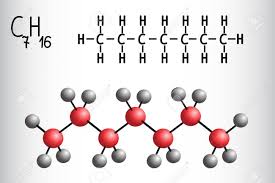
Heptane is the straight-chain alkane with the chemical formula C7H16, and is one of the main components of gasoline (petrol). When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and isooctane which is expressed as the percentage of isooctane in heptane and is listed on pumps for gasoline (petrol) dispensed globally. Its appearance is as follows:

Figure 1 Appearance of Heptane.
Application
Heptane is widely used in laboratories as a non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve an oil spot to show the previous presence of organic compounds on a stained paper [1]. This is done by shaking the stained paper in a heptane solution for about half a minute.Aqueous bromine may be distinguished from aqueous iodine by its appearance after extraction into heptane. In water, both bromine and iodine appear brown. However, iodine turns purple when dissolved in heptane, whereas the bromine solution remains brown.
Heptane is commercially available as mixed isomers for use in paints and coatings, as the rubber cement solvent "Bestine", the outdoor stove fuel;"Powerfuel" by Primus, as pure n-heptane for research and development and pharmaceutical manufacturing and as a minor component of gasoline.
Heptane is also used as an adhesive remover by stamp collectors. Since 1974, the United States Postal Service has issued self-adhesive stamps that some collectors find difficult to separate from envelopes via the traditional method of soaking in water. Heptane-based products like Bestine, as well as limonene-based products, have become popular solvents for removing stamps more easily.[2]
Heptane is defined as the zero point of the octane rating scale [3]. It is a lighter component in gasoline, burns more explosively, causing engine pre-ignition (knocking) in its pure form, as opposed to octane isomers, which burn more slowly and give less knocking. It was originally chosen as the zero point of the scale because of the availability of very high purity n-heptane, unmixed with other isomers of heptane or other alkanes, distilled from the resin of Jeffrey pine and from the fruit of Pittospo"rum resiniferum. Other sources of heptane and octane, produced from crude oil, contain a mixture of different isomers with greatly differing ratings, and do not give as precise a zero point.
Preparation
The linear heptane can be obtained from Jeffrey pine oil [4]. The six branched isomers without a quaternary carbon can be prepared by creating a suitable secondary or tertiary alcohol by the Grignard reaction, converting it to an alkene by dehydration, and hydrogenating the latter. The 2,2-dimethylpentane isomer can be prepared by reacting tert-butyl chloride with n-propyl magnesium bromide. The 3,3-dimethylpentane isomer can be prepared from tert-amyl chloride and ethyl magnesium bromide.
Toxicity
Acute exposure to heptane vapors can cause dizziness, stupor, incoordination, loss of appetite, nausea, dermatitis, chemical pneumonitis, or unconsciousness, possible peripheral neuropathy [5]. Inhalation of 1,000 ppm for 6 minutes was associated with slight dizziness. Exposure to 5,000 ppm for 4 minutes produced complaints of nausea, a loss of appetite, vertigo, and incoordination. A 15-minute exposure to 5,000 ppm produced a state of intoxication characterized by uncontrolled hilarity in some individuals and in others a stupor lasting for 30 minutes after the exposure. A 15-minute exposure to 5,000 ppm produced a state of intoxication characterized by uncontrolled hilarity in some individuals and in others a stupor lasting for 30 minutes after the exposure. According to Patty [6], a 4-minute exposure to this same concentration produces vertigo and incoordination. These symptoms described by Patty could perhaps impede escape [6].
References
[1]Hofmann. On the action of trichloride of phosphorus on the salts of the aromatic monamines. Proceedings of the Royal Society of London. 1867, 15: 54–62.
[2]Butler. It's Like Magic: Removing Self-Adhesive Stamps from Paper. American Philatelic Society. 2020, 5(4): 123-134.
[3]Dymond et al. Viscosity of Selected Liquid n‐Alkanes. Journal of Physical and Chemical Reference Data.1994, 23 (1): 41–53.
[4]Graham et al. The preparation and properties of the isomeric heptanes. Part I. Preparation. Journal of the American Chemical Society, 1929, 51(5): 1483–1491.
[5]Patty et al. Odor intensity and symptoms produced by commercial propane, butane, pentane, hexane, and heptane vapor. Pittsburgh, PA: Department of Commerce, U.S. Bureau of Mines, Report of Investigations, 1929, No. 2979: 1–10.
[6]Patty et al. Industrial hygiene and toxicology. 2nd rev. ed. Vol. II. Toxicology. New York, NY: Interscience Publishers, Inc., 1963, 1198-1199.
You may like
Related articles And Qustion
Lastest Price from Heptane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 142-82-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 142-82-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons