Toxicity of Nonylphenol ethoxylate
General description
Nonylphenol ethoxylate (NPE) is the most important alkylphenol ethoxylate. NPE accounts for approximately 85% of alkylphenol ethoxylate production (1). An NPE is composed of a nonyl chain, usually branched, attached to a phenol ring (hydrophobe moiety) which is combined, via an ether linkage, with one or more ethylene oxide or polyoxyethylene units (hydrophile moiety).
A particular NPE is identified by the length of the polyoxyethylene chain (average number of ethylene oxide units, abbreviated EO). NPEs of a particular average ethylene oxide chain length, produced by different manufacturers, may differ in ranges of ethylene oxide chain lengths.
NPEs with between 1 and 13 ethylene oxide units are liquid. NPEs with 14 and 15 ethylene oxide units are paste-like liquids. Viscosity increases with increasing ethylene oxide chain length, and NPEs with 20 or more ethylene oxide units are waxy solids. Color varies from colorless to light amber. NPEs with higher numbers of ethylene oxide units are opaque. (2).
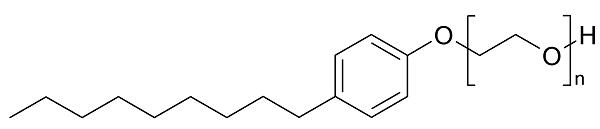
Figure 1 Molecular formula of Nonylphenol ethoxylates
Application
NPEs are produced for high-volume use in many industrial sectors, including industrial laundering, textile processing, pulp and paper processing, paint and resin formulation, oil and gas recovery, steel manufacturing, pest control and power generation. NPEs are also utilized in the production and formulation of many commercially sold products: as an industrial and commercial detergent, as an emulsifier in wax for fruit and vegetables, as a polymer resin in plastic food packaging and polyethylene plastic, in cosmetic products (such as skin cream, deodorant, makeup, hair dye, and shampoo), and even in spermicides (3-5).
The largest quantity of NPEs is used in cleaning products, especially detergents (3-4) Of the 260 million pounds of NP used in 2004, 80% was used as a surfactant. In general, 37% of NPE metabolites enter the aquatic ecosystem (3-4). Based on this data, nearly 77 million pounds of NPE-based cleaning agents entered U.S. waterways in 2004. U.S. demand for all surfactants in 2007 was 7.5 billion pounds (6). U.S. and Canadian consumption of NPE surfactants has been estimated between 300 and 400 million pounds per year (7). In a study by ToxEcology in 2002, NPE surfactant consumption in Canada was estimated at approximately 29 million pounds. It is not clear if this estimate includes soap, so it may be an underestimate of total surfactant consumption by Canada. However, if Canadian consumption of NPEs is approximately 29 million pounds, then U.S. consumption of NPEs would range from approximately 270 to 370 million pounds.
Synthesis
NPEs are manufactured by reacting NP(Nonylphenol) with ethylene oxide. A polyethylene oxide chain of any desired length can be built up by continued introduction of ethylene oxide into the reaction mixture. Such a reaction yields NPEs with a mixture of ethylene chain lengths, and the number of ethylene units used to describe the product is the average number (8). During base catalyzed ethoxylation of nonylphenol, ethylene oxide preferentially reacts with the free nonylphenol and only when this has all reacted do longer ethylene oxide chains form. Nonylphenol ethoxylates have much narrower homologue distributions than alcohol ethoxylates.
880 million pounds (4 million metric tons) of alkyl phenol ethoxylates are used annually throughout the world. 80-85% are sold as nonylphenol ethoxylate (NPE), over 15% as octylphenol ethoxylate, and 1% each as dinonylphenol and dodecylphenol ethoxylates (1).
Environmental fate
Nonylphenol is a xenobiotic compound used in the manufacture of antioxidants, lubricating oil additives and the production of nonylphenol ethoxylates surfactants which is its major use (65%) (9). Nonylphenol ethoxylates are highly cost and effective surfactants with exceptional performance and consequently used widely in industrial, institutional, commercial and household applications such as detergents, emulsifiers, wetting and dispersing agents, antistatic agents, demulsifiers and solubilizers. Due to the extensive use of nonylphenol ethoxylates, they reach sewage treatment works in substantial amounts where they are incompletely degraded to nonylphenol.
NPEs may degrade into nonylphenol. During degradation NPEs’ ethylene oxide units are cleaved off the ethylene oxide chain until only short-chain NPE remain, typically mono- and diethylene oxides. Oxidation of these oligomers creates the corresponding carboxylic acids. This leaves several degradation products: short-chain ethoxylates, their carboxylic acids, and nonylphenols (1). The rate of biodegradation seems to decrease with increasing length of the ethylene oxide chain (10).
Due to its physical–chemical characteristics, such as low solubility and high hydrophobicity, nonylphenol accumulates in environmental compartments that are characterized by high organic content, typically sewage sludge and river sediments, where it persists (11). The occurrence of nonylphenol in the environment is clearly correlated with anthropogenic activities such as wastewater treatment, landfilling and sewage sludge recycling. Nonylphenol is found often in matrices such as sewage sludge, effluents from sewage treatment works, river water and sediments, soil and groundwater. The impacts of nonylphenol in the environment include feminization of aquatic organisms, decrease in male fertility and the survival of juveniles at concentrations as low as 8.2 μg/l (11). Due to the harmful effects of the degradation products of nonylphenol ethoxylates in the environment, the use and production of such compounds have been banned in EU countries and strictly monitored in many other countries such as Canada and Japan. Although it has been shown that the concentration of nonylphenol in the environment is decreasing, it is still found at concentrations of 4.1 μg/l in river waters and 1 mg/kg in sediments. Nonylphenol has been referred to in the list of priority substances in the Water Frame Directive and in the 3rd draft Working Document on Sludge of the EU. Consequently, there is currently a concern within some industries about the possibility of future regulations that may impose the removal of trace contaminants from contaminated effluents (11).
Toxicity
The possible routes of human exposure to NPEs are dermal contact and inhalation by workers involved in the manufacture and use, dermal and inhalation exposure of consumers from household pesticide products, dermal contact to cleaning products and cosmetics, mucous membrane contact to spermicides; inhalator exposure via the environment through air, and oral exposure via the environment through drinking water and food.
Cosmetic formulations containing NPEs with 4, 9, or 12 ethylene oxide units in varying concentrations have been tested for skin irritation in human volunteers (2). The results show a range of effects from no to mild skin irritation.
NPEs with 4, 9, 15, or 50 ethylene oxide units were non-sensitizing in human test subjects (2). A 10% NPE spermicidal film caused mild local vaginal irritation in 2 out of 30 women (2).
References
1. Anonymous (1997). APE producers offering data, co-operation. INFORM 12, 1271-1279.
2. CIR (Cosmetic Ingredient Review (1983). Final report on the safety assessment of nonoxynols -2,-4,-8,-9,-10,-12,-14, -15, -30, -40, and -50. J Am Coll Toxicol 2, 35-60.
3. Environment Canada. 2001. Nonylphenol and Its Ethoxylates: Priority Substances List Assessment Report. Minister of Public Works and Government Services.
4. Environment Canada. 2002. Canadian Environmental Quality Guidelines for Nonylphenol and its Ethoxylates (Water, Sediment, and Soil). Scientific Supporting Document. Ecosystem Health: Sciencebased Solutions Report No. 1-3. National Guidelines and Standards Office, Environmental Quality Branch, Environment Canada, Ottowa.
5.European Union. 4-Nonylphenol (branched) and Nonylphenol Risk Assessment Report. Institute for Health and Consumer Protection, European Chemicals Bureau, Vol.10.
6.Rust and Wildes. 2008. Surfactants A Market Opportunity Study Update. Prepared for the United Soybean Board. Omni Tech International, Ltd. December.
7. EPA. 2008. Bi-national Framework for Identifying Substances of Potential Threat to The Great Lakes Basin, Test Case: Nonylphenol and its Ethoxylates (NPEs). Draft, November 20, 2008.
8.Swisher RD (1970). Surfactant biodegradation. Marcel Dekker, Inc., New York
9. USEPA (United States Environmental Protection Agency). Testing consent order on 4-nonylphenol, branched. Fed Reg 1990;35:5991–4.
10. Talmage SS (1994). Environmental and human safety of major surfactants. Alcohol ethoxylates and alkylphenol ethoxylates. The soap and detergent association. Lewis Publishers. Boca Raton, Ann Arbor, London, Tokyo.
11. Soares A , Guieysse B , Jefferson B , et al. Nonylphenol in the Environment: A Critical Review on Occurrence, Fate, Toxicity and Treatment in Wastewaters[J]. Environment International, 2008, 34(7):1033-1049.