Methicillin: Phenoxypenicillins: Antimicrobial Activity, Susceptibility, Administration and Dosage Clinical Uses etc.
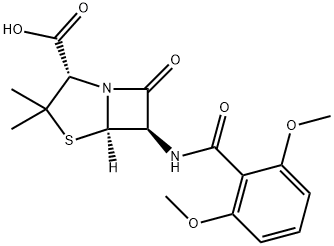
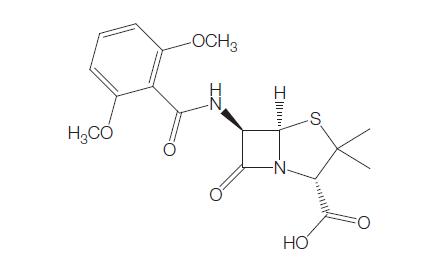
Methicillin was the first penicillinase-resistant semi-synthetic penicillin to be derived from the penicillin nucleus, 6-aminopenicillinic acid (6-APA) (Knudsen and Rolinson, 1960). The drug was discovered at Beecham Research Laboratories. Initially it was used widely, but because of its toxicity, it was gradually superseded by other penicillinase-resistant penicillins, such as nafcillin, oxacillin, cloxacillin, dicloxacillin, and flucloxacillin. Methicillin is now no longer marketed for human use. The chemical structure is shown in Figure 4.1.
ANTIMICROBIAL ACTIVITY
a. Routine susceptibility
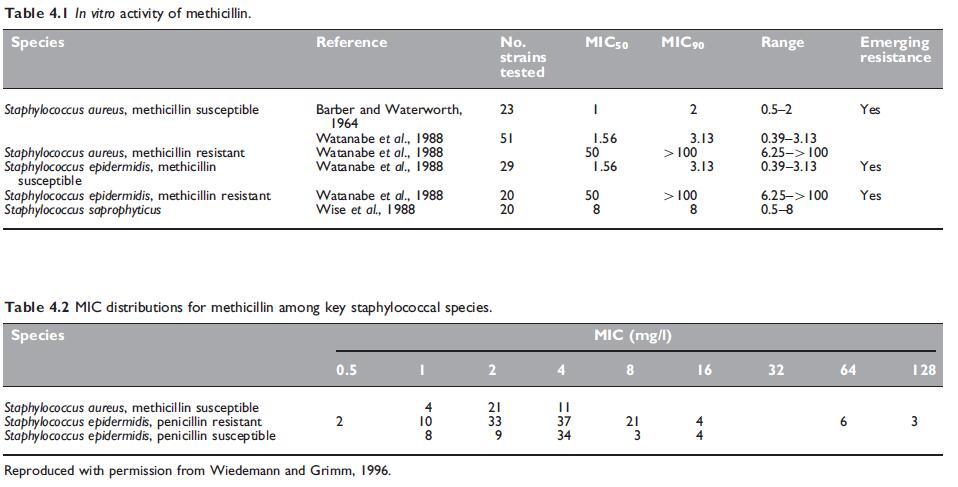
The activity of methicillin is similar to that of penicillin G (see Chapter 1, Benzylpenicillin (Penicillin G)). Methicillin is active against Grampositive bacteria and also against Gram-negative cocci, such as meningococci and gonococci. Gonococci with altered penicillin-binding proteins have elevated minimum inhibitory concentrations (MICs) to methicillin, as well as penicillin G. Methicillin is some 20- to 50-fold less active than penicillin G against bacteria susceptible to both drugs. Being both stable and active in the presence of staphylococcal b-lactamase (Rolinson et al., 1960), methicillin is active against penicillin G-resistant staphylococci. Stability of the penicillinase-resistant penicillins in the presence of the enzyme is one of degree. Methicillin and nafcillin are the most stable and they are followed closely in descending order of stability by dicloxacillin, oxacillin, cloxacillin, and flucloxacillin (Sabath et al., 1975; Basker et al., 1980). Reported results on such stability are conflicting and this property is of doubtful clinical significance.
b. Emerging resistance and cross-resistance
Methicillin-resistant Staphylococcus aureus
Staphylococcal resistance to methicillin is not due to the destruction of the antibiotic by a bacterial enzyme such as a b-lactamase, but it is acquired in a different manner. There is no penetration barrier to methicillin in methicillin-resistant S. aureus (MRSA) strains. Instead resistance is mediated through the acquisition of a gene that encodes an additional penicillin-binding protein (PBP), PBP2a (sometimes designated PBP2u), which performs the functions of PBP2, but has much lower affinity for methicillin and most other b-lactams (Stapleton and Taylor, 2002). PBP2a is a major enzyme involved in the transpeptidation step of peptidoglycan cross-linking in the cell wall. S. aureus has four penicillin-binding proteins, PBP1, PBP2, PBP3, and PBP4.
PBP2a is encoded by the gene mecA. This gene is always found inside specific gene complexes inserted into the staphylococcal chromosome. The gene complexes are named SCCmec (staphylococcal cassette chromosome mec), and so far five distinct types designated SCCmec I–V have been described. SCCmec is a mobile genetic element. Each SCCmec contains specific ccr genes that encode recombinase enzymes, allowing for the excision and integration of the cassette into the staphylococcal chromosome (Deurenberg et al., 2006).
Methicillin-tolerant S. aureus
These strains have a deficiency of autolytic enzymes in their cell walls, the presence of which is necessary for the penicillins to exert a bactericidal effect. The MICs of methicillin against these strains are in the usual low (susceptible) range, but minimum bactericidal concentrations (MBCs) are high (Sabath et al., 1977).
Methicillin-resistant coagulase-negative staphylococci Resistance to methicillin is a common finding in a range of coagulasenegative staphylococci. Among the common species associated with human disease, namely S. epidermidis, S. saprophyticus, S. haemolyticus, and S. hominis, S. saprophyticus has natural reduced susceptibility to methicillin and the antistaphylococcal penicillins.
MECHANISM OF DRUG ACTION
Methicillin, like other penicillins, inhibits peptidoglycan synthesis by inhibiting the transpeptidase enzymes PBP1a, 1b, and 2.
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Methicillin is unstable in acids, so it is ineffective if given orally. Methicillin can be administered i.m. or i.v., and the i.v. route is useful to avoid frequent painful i.m. injections. The dose can be varied widely according to the site and severity of infection. For infections of moderate severity, a commonly used adult dosage is 1 g 4-hourly. For serious infections, the dose is often doubled or increased even further. Daily doses of up to 25 g have been given i.v. for several weeks without toxic effect.
b. Newborn infants and children
The usual dose of methicillin for children is 100 mg/kg body weight per day, given in four or six divided doses. Renal excretion of methicillin in newborn infants is decreased. A dose of 25 mg/kg body weight every 12 hours should be given to infants weighing less than 2000 g and who are 0–7 days old. For infants still weighing less than 2000 g, but who are 8–30 days old, the dose of 25 mg/kg should be given 8-hourly. For infants weighing more than 2000 g, the dose is 25 mg/kg given 8-hourly to those aged 0–7 days, and the same dose 6-hourly to those aged 8–30 days (McCracken, 1974; McCracken and Nelson, 1983). After the age of one month, methicillin should be administered in doses recommended for older children.
c. Altered dosages
Impaired renal function
Methicillin, being relatively nontoxic, is often administered in the normal dosage to patients with mild to moderate renal failure. Because its elimination half-life is prolonged by profound renal failure, the dosage should be reduced and an adult dose of 1–2 g administered every 8–12 hours has been recommended (Bulger et al., 1964). If a patient with renal failure requires i.v. methicillin in large doses (equivalent to 16 g daily for an adult with normal renal function), this dosage can be adjusted in a similar manner to that of penicillin G. Dosage can be estimated by substituting 0.6 g of methicillin for 0.6 g of penicillin G.
PHARMACOKINETICS AND PHARMACODYNAMICS
a. Bioavailability
Methicillin is not absorbed when administered orally. The estimated elimination half-life of methicillin is around 30 minutes. Methicillin has relatively low protein binding to serum albumin. Depending on the type of assay binding ranges from 37% to 49% (Craig and Suh, 1991).
b. Drug distribution
After a 1-g i.m. dose, a mean peak serum level of 18mg/l is reached after 30 minutes, and this level falls to 3–4mg/l after 3 hours (Knudsen and Rolinson, 1960). After an i.v. injection of 1 g methicillin over a 5-minute period, a peak serum level of about 60mg/l is reached. This peak level is doubled by doubling the dose. Subsequently, there is a rapid fall in the serum level to about 7mg/l after 1 hour, and after 2–3 hours the level is usually less than 1mg/l. Usually methicillin cannot be detected in the serum after 4 hours.
c. Clinically important pharmacokinetic and pharmacodynamic features
The clinically important pharmacokinetic and pharmacodynamic features of methicillin are as for nafcillin (see Chapter 6, Nafcillin).
d. Excretion
Methicillin is excreted in urine in an unchanged active form (Stewart et al., 1960). Very high urine concentrations of methicillin are attained, provided renal function is normal. It is excreted by both glomerular filtration and tubular secretion, and up to 80% of an injected dose can be recovered from urine. Probenecid delays renal tubular secretion.
TOXICITY
Adverse reactions to methicillin, particularly renal reactions, are more common than with other antistaphylococcal penicillins. For this reason, methicillin is no longer marketed for human use.
a. Hypersensitivity reactions
These should be anticipated in patients known to be sensitive to the penicillins. However, not all patients allergic to penicillin G react to methicillin. In a study of eight consecutive patients with histories of penicillin G anaphylaxis, Luton (1964) showed that all tolerated usual i.m. doses of methicillin without reaction. Antecedent skin testing with penicillin G in these patients gave positive reactions, while similar tests with methicillin were negative. Skin testing with these reagents is not recommended. Severe allergic reactions to methicillin and to other semisynthetic penicillins may be less common than to penicillin G. Nevertheless, it should be assumed that patients allergic to other penicillins will be sensitized to methicillin, and it should be avoided in such subjects.
b. Drug fever
This can occur with methicillin. It is abrupt in onset and the patient usually appears otherwise relatively well. It rapidly resolves when the drug is stopped, and may recur later if another penicillin analog is administered (Yow et al., 1976).
c. Nephrotoxicity
An interstitial nephritis can be caused by large i.v. doses of methicillin (Baldwin et al., 1968; Woodroffe et al., 1974; Galpin et al., 1978). This is characterized by fever, rash, eosinophilia, hematuria, proteinuria, sterile pyuria, marked eosinophiluria, and renal insufficiency. Microscopic changes in the kidneys consist of patchy but usually heavy interstitial infiltrate with lymphocytes, plasma cells, and eosinophils,i.e. interstitial changes without glomerular abnormalities. Cogan and Arieff (1978) reported a patient in whom methicillin-induced interstitial nephritis also resulted in functional impairment specific for the distal tubule.
d. Hematologic side-effects
Leukopenia is fairly common during methicillin therapy. It was observed in 16 of 124 children who received methicillin for 10 days or longer, and it usually occurred 10–20 days after starting treatment (Yow et al., 1976). Some patients developed a decrease in both neutrophils and lymphocytes, others an absolute neutropenia (less than 500/mm3). The white cell count usually reverted to normal in a few days after cessation of methicillin. The leukopenia may worsen if another penicillin, such as oxacillin, is substituted for methicillin. No serious problems resulting from leukopenia have been encountered.
e. Abnormal liver function
As with the isoxazolyl penicillins, elevation of serum glutamic oxaloacetic transaminase has been occasionally observed during methicillin therapy (Berger and Potter, 1977).
f. Encephalopathy
This complication, similar to that seen after ‘‘massive’’ doses of penicillin G, would be expected if very large doses (50–100 g daily) were given i.v., and it could arise with smaller doses in patients with renal failure.
CLINICAL USES OF THE DRUG
Methicillin is now no longer marketed for human use, but was previously widely used to treat staphylococcal infections.
a. S. aureus infections
Methicillin is useful for the treatment of S. aureus infections (proven or suspected) due to b-lactamase-producing staphylococci which are resistant to penicillin G. Originally, this antibiotic was regarded as the drug of first choice for severe staphylococcal infections, such as septicemia, endocarditis, pneumonia, meningitis, osteomyelitis, and septic arthritis.
It is effective for the treatment of penicillin-resistant staphylococcal infections (White and Varga, 1961), including staphylococcal septicemia and endocarditis (Allen et al., 1962). It was extensively used for these infections for some years after its discovery. All other parenteral b-lactamase-resistant penicillins, such as oxacillin, cloxacillin, dicloxacillin, flucloxacillin, and nafcillin, have the same therapeutic efficacy as methicillin (Neu, 1982). The drug which is selected is therefore usually the one with which the clinician is most familiar.
b. Staphylococcus epidermidis infections
Methicillin was used for severe hospital-acquired S. epidermidis infections, such as prosthetic valve endocarditis, provided that the strain was sensitive. For the reasons given above, one of the isoxazolyl penicillins or nafcillin are now preferred for these infections. Methicillin, other penicillinase-resistant penicillins, and cephalosporins are no longer suitable for immediate emergency treatment of severe hospital-acquired S. epidermidis infections, as >50% of hospital strains are methicillin resistant. Vancomycin is the drug of choice, perhaps combined with rifampicin (Karchmer et al., 1983).
References
Allen JD, Roberts CE, Kirby WMM (1962). Staphylococcal septicaemia treated
with methicillin: Report of twenty-two cases. N Engl J Med 266: 111.
Archer GL, Niemeyer DM, Thanassi JA, Pucci MJ (1994). Dissemination
among staphylococci of DNA sequences associated with methicillin
resistance. Antimicrob Agents Chemother 38: 447.
Baldwin DS, Levine BB, McCluskey RT, Gallo RR (1968). Renal failure and
interstitial nephritis due to penicillin and methicillin. N Engl J Med 279:
1245.
Basker MJ, Edmondson RA, Sutherland R (1980). Comparative stabilities of
penicillins and cephalosporins to staphylococcal beta-lactamase and
activities against Staphylococcus aureus. J Antimicrob Chemother 6: 333.
Berger M, Potter DE (1977). Pitfall in diagnosis of viral hepatitis on
haemodialysis unit. Lancet 2: 95–6.
Border WA, Lehman DH, Egan JD et al. (1974). Antitubular basementmembrane
antibodies in mbethicillin-associated interstitial nephritis. N Engl J
Med 291: 381.
Boyle-Vavra S, Daum RS (2007). Community-acquired methicillin-resistant
Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab Invest
1: 3.
Bracis R, Sanders CV, Gilbert DN (1977). Methicillin hemorrhagic cystitis.
Antimicrob Agents Chemother 12: 438.
Bulger RJ, Lindholm DD, Murray JS, Kirby WMM (1964). Effect of uremia on
methicillin and oxacillin blood levels. JAMA 187: 319.
Chambers HF, Archer G, Matsuhashi M (1989). Low-level methicillin
resistance in strains of Staphylococcus aureus. Antimicrob Agents Chemother
33: 424.
Cogan MC, Arieff AI (1978). Sodium wasting, acidosis and hyperkalemia
induced by methicillin interstitial nephritis. Evidence for selective distal
tubular dysfunction. Am J Med 64: 500.
Couto I, de Lencastre H, Severina E et al. (1996). Ubiquitous presence of a
mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug
Resist 2: 377.