Ampicillin and amoxicillin: Mechanism of Drug Action, Pharmacokinetics, Pharmacodynamics, and Clinical Uses
Ampicillin and amoxicillin have actions similar to penicillin G (Benzylpenicillin (Penicillin G)). The differences in their antibacterial spectrum, compared with penicillin G, can be explained by their greater ability to penetrate the outer membrane of the cell wall of some Gram-negative bacilli.
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Ampicillin
AMP is acid stable and can be given orally. It is available as capsules (250 and 500 mg), pediatric tablets (125 mg), syrup (5 ml containing 125 or 250 mg), and in vials (250 mg, 500 mg, and 1 g) suitable for parenteral administration. The usual oral dosage for children and adults to treat mild to moderate infections is 50–100 mg/kg body weight per day, given in four divided doses. A common oral adult dosage is 0.5 g 6-hourly, although 250 mg 6-hourly may suffice for mild infections due to highly susceptible organisms.
Amoxicillin
AMOX is available as capsules (125, 250, and 500 mg), dispersible tablets (3 g), oral suspension (5 ml containing 125 or 250 mg), and in vials of sodium amoxicillin (250mg, 500 mg, and 1 g) suitable for parenteral administration. The usual oral dosage of amoxicillin is 50–100 mg/kg body weight per day, administered in three or four divided doses. The usual adult dosage of this drug is 250–500 mg, given 6- or 8-hourly.
b. Newborn infants and children
Ampicillin
Pediatric preparations include pediatric tablets (125 mg) and syrup (5 ml containing 125 or 250 mg), as well as the parenteral preparations. The usual oral AMP dosage for children to treat mild to moderate infections is 50–100 mg/kg body weight per day, given in four divided doses.
For parenteral administration of AMP in serious infections, high doses are often necessary; in children a daily dose of 150–200 mg/kg is recommended, but up to 400 mg/kg/day has been given occasionally (Fleming et al., 1967).
Amoxicillin
Similar to adults, the usual oral AMOX dose for children is is 50– 100 mg/kg body weight per day, administered in three or four divided doses For serious infections, the parenteral dosage is 150–200 mg/kg body weight per day, given in six divided doses.
c. Altered dosages
Ampicillin
Impaired renal function
AMP is relatively nontoxic and is often given in the usual doses to these patients. If high parenteral doses are used, appropriate dosage reduction is necessary. In anuric patients, the AMP serum half-life is prolonged to 8.5 hours. In patients with severe renal failure, it has been suggested that the total daily dose should be halved and given in two divided doses (Bennett et al., 1970). Others consider that an AMP dose of 0.5 g daily may be sufficient for patients with a creatinine clearance of 10 ml/min or less (Lee and Hill, 1968).
Amoxicillin
Impaired renal function
Amoxicillin is relatively nontoxic and may be given in the usual recommended dosage to patients with mild renal failure. In patients with moderate to severe renal failure, as in the case of ampicillin, the dose should be reduced (Sabto et al., 1973). With amoxicillin, dosage adjustment is unnecessary if the patient’s creatinine clearance exceeds 30 ml/min; with creatinine clearance of 10–30 ml/min and o10 ml/ min, the dose should be halved or quartered, respectively. Amoxicillin is removed from the body by hemodialysis, but removal by peritoneal dialysis is slow. In anuric or end-stage renal failure patients who are treated by regular hemodialysis, a 1-g i.v. dose of amoxicillin should be given at the end of dialysis, and then be repeated once every 23 hours (Chelvan et al., 1979; Humbert et al., 1979).
PHARMACOKINETICS AND PHARMACODYNAMICS
a. Bioavailability
Ampicillin
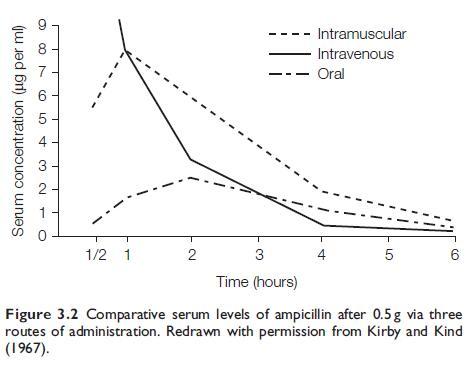
After oral administration of AMP to adults, peak serum concentrations
are obtained at about 2 hours, and the drug is still detectable in
the serum at 6 hours (see Figure 3.2). Doubling the dose virtually
doubles the serum concentration. The absorption of AMP is reduced
by about 50% if it is administered with food (Welling and Tse, 1982).
Concurrent administration of cimetidine does not diminish AMP
absorption (Rogers et al., 1980), but co-administration with chloroquine
does (Ali, 1985). The drug is absorbed normally in patients
with celiac disease (Parsons et al., 1975). The concomitant use of
Lactobacillus preparations does not interfere with the absorption of
ampicillin (Yost and Gotz, 1985).
With i.m. administration, a much higher peak level is achieved
within 30 minutes (see Figure 3.2). AMP, compared with penicillin G,
produces considerably higher serum levels after parenteral administration;
this is chiefly due to its slower renal clearance (Kirby and
Kind, 1967).
Amoxicillin
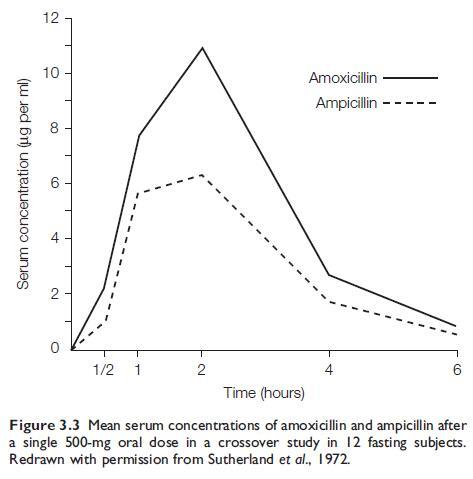
Amoxicillin is well absorbed after oral administration (Sutherland et al., 1972; Verbist, 1976). After a 0.5-g dose in adults, a peak serum level of 8–10 mg/ml is reached 2 hours later (see Figure 3.3). Doubling the dose doubles the peak serum level. Thereafter, serum concentrations fall and reach zero after 6–8 hours. These serum levels are about twice as high as those produced by an equivalent dose of oral AMP,and about the same as those attained when this dose of AMP or AMOX is given i.m. (Hill et al., 1980). The absorption of amoxicillin appears to be an active process.
b. Drug distribution
Ampicillin
AMP is evenly distributed throughout most body tissues. With the exception of the kidney and liver, tissue concentrations are lower than simultaneous serum levels. In healthy humans, the intrarenal tissue concentration is about 8-fold higher than the concomitant serum level. By contrast, the AMP concentration in chronic glomerulonephritic kidneys is only about half the serum level at the time. In such severe kidney disease, the urinary concentration of AMP is greatly reduced, and the serum rather than urinary concentration more closely reflects the AMP renal tissue level (Whelton et al., 1972). Animal studies show that AMP renal tissue levels are also significantly reduced in acute pyelonephritis (Trottier and Bergeron, 1981).
Amoxicillin
Amoxicillin is probably largely distributed similar to AMP, but some minor differences have been described. In a comparison with AMP, May and Ingold (1974) found that both drugs produced similar concentrations in purulent sputum, but levels of amoxicillin were higher in mucoid sputum. Law et al. (1983) used amoxicillin at a dosage of 0.5 g three times daily to treat infective exacerbations of chronic bronchitis in adults.
c. Clinically important pharmacokineticb and pharmacodynamic features
The clinically important pharmacokinetic and pharmacodynamic features of both AMP and AMOX are similar to those of other betalactams, such as penicillin G. The pharmacokinetics and pharmacodynamics of the beta-lactam antibiotics have been summarized by Craig (1998) and DeRyke et al. (2006). These include the fact that bacterial killing is not dose-related (as is the case with aminoglycosides), the postantibiotic effect of beta-lactam agents is short, and the time for which serum concentrations are above the MIC is important for eradication of infecting bacteria. Thus, for serious infections, continuous infusion or frequent intravenous bolus administration, is likely to be most effective.
Excretion
Ampicillin
Approximately 30% of an oral dose of AMP is excreted in urine during the first 6 hours. High concentrations of the active drug are attained in urine; after a 0.5-g oral dose, urinary concentrations range from 250 to 1000 mg/ml. There are no active AMP metabolites formed in the body prior to excretion (Cole and Ridley, 1978). AMP is excreted partly by glomerular filtration and partly by tubular secretion. Probenecid slows excretion by partial blockage of tubular secretion. After parenteral administration, about 75% of the dose is excreted in urine. Compared with penicillin G, AMP is cleared at a slower rate by the kidney, its serum half-life in normal adults being 1.5 hours, compared with 0.5 hours for penicillin G. Therefore, the action of probenecid in lowering renal clearance and elevating serum levels is more marked with penicillin G (Kirby and Kind, 1967; Bennett et al., 1974).
Amoxicillin
About 58–68% of an orally administered dose of AMOX is excreted in the urine in an unchanged active form during the first 6 hours (Sutherland et al., 1972). High AMOX urinary levels of 115–1850 mg/ml occur after a 0.5-g oral dose to adults. Like AMP, this drug is excreted by both glomerular filtration and tubular secretion; the latter can be reduced by concomitant administration of probenecid (Neu, 1979).
e. Drug interactions
Ampicillin
Similar to penicillin G, probenecid slows excretion by partial blockage of tubular secretion. Concurrent administration of cimetidine does not diminish AMP absorption (Rogers et al., 1980), but co-administration with chloroquine does (Ali, 1985). Animal experiments show that morphine administration elevates the serum levels of AMP after its i.v. administration. Morphine appears to impair both renal and hepatobiliary elimination of AMP. In contrast, morphine markedly reduces the drugs’ levels in serum, when it is given by gastric intubation. This results from delayed absorption because of retardation of gastric emptying by morphine (Garty and Hurwitz, 1985).
Amoxicillin
Drug–drug interactions for AMOX are generally similar to those of AMP.
TOXICITY
In general, AMP and AMOX have similar profiles in terms of adverse reactions.
a. Hypersensitivity reactions and rashes
AMP and AMOX are ‘cross-allergenic’ with other penicillins, and in sensitized patients they may evoke any of the hypersensitivity reactions caused by penicillin G. Patients allergic to penicillin G do not always react to AMP–AMOX, but because of this possibility AMP–AMOX are contraindicated in such patients. Despite this, on rare occasions AMP has been used when it has been the drug of choice for the treatment of a severe infection. Petheram and Boyce (1976) used i.v. AMP plus gentamicin successfully for the treatment of E. faecalis endocarditis in a penicillin-allergic patient; prednisolone and an antihistamine were administered concomitantly and full resuscitation facilities were immediately available.
Rashes are very common in patients with infectious mononucleosis (IM) who are given AMP–AMOX. A small percentage of IM patients develop a rash without a history of prior drug therapy, but it has been estimated that 65–95% of them will develop a rash if they are given AMP (Pullen et al., 1967; Lund and Bergan, 1975). AMP had been prescribed frequently for pharyngitis, a condition for which other forms of penicillins are indicated.
b. Gastrointestinal side-effects
Oral or, less commonly, parenteral AMP therapy can cause nausea and diarrhea, but these are usually not serious (Bass et al., 1973). In one study of children, the severity of diarrhea was such that 85 (8%) of all orally treated and 3% of all i.v.-treated patients required discontinuation of AMP or the use of antidiarrhea drugs (Phillips et al., 1976). AMP-induced diarrhea appeared to be more common in younger children. In another study of children treated for otitis media, AMP oral suspension caused diarrhea more frequently than amoxicillin or co-trimoxazole suspensions (Feder, 1982). In adults, diarrhea may occur in 5–20% of treated patients and is probably more common in older age groups (Gurwith et al., 1977; Lusk et al., 1977).
c. Hepatic damage
AMP, like other penicillins, can cause liver injury including cholestatic hepatitis (Koklu et al., 2003).
d. Nephropathy
A number of anecdotal case reports indicate that AMP is an uncommon cause of renal damage. One case of interstitial nephritis, which immediately followed a small dose of AMP, has been reported (Tannenberg et al., 1971). Azotemia did not occur, but the other clinical, histologic, and immunologic features were similar to those of methicillin nephritis (see Chapter 4, Methicillin). Maxwell et al. (1974) described another patient with AMP-induced interstitial nephritis in whom the large dose of the drug used (200 mg/kg/day) was a probable factor. Lee and Hill (1968) observed three patients with renal failure who, following a severe cutaneous AMP hypersensitivity reaction, developed further permanent deterioration in renal function.
e. Hematologic side-effects
Similar to penicillin G, AMP, if administrated in large doses i.v., can cause neutropenia, and this is more likely to occur in patients with preexisting liver disease. Agranulocytosis with monohistiocytosis has been described in one patient in association with AMP therapy (Graf and Tarlov, 1968). Severe acute thrombocytopenia with bleeding occurred in one patient receiving AMP and no other agents could be implicated (Brooks, 1974). With high doses of the drug, there is a tendency for red cells to become coated with AMP and a Coombs-positive hemolytic anemia may be a sequel, as with penicillin G (see Chapter 1, Benzylpenicillin (Penicillin G)).
f. Encephalopathy
Convulsions may be anticipated with very large doses of i.v. AMP, particularly if serum levels reach 800 mg/ml. This threshold concentration for convulsions is higher than that for penicillin G. AMP may cause convulsions when administered in ordinary does, such as 2 g daily, if it is given to patients prone to seizures, such as those with head injuries or idiopathic epilepsy (Serdaru et al., 1982). Encephalopathy may also occur if large doses of amoxicillin are given intravenously.
CLINICAL USES
a. Group A streptococcal pharyngitis
Studies have shown that amoxicillin is as efficacious as, and may be superior to, penicillin V in the treatmen ot these infections (Gopichand et al., 1998; Curtin-Wirt et al., 2003). The superior efficacy may be due to inadequate doses of penicillin V to achieve effective tissue concentrations. Amoxicillin given once daily is as effective as amoxicillin administered twice daily (Clegg et al., 2006) for the treatment of group A streptococcal pharyngitis and is of equal efficacy as twice-daily phenoxymethylpenicillin for this indication (Lennon et al., 2008). However, amoxicillin should not be used for pharyngitis/ tonsillitis unless infectiousb mononucleosis has been excluded.
b. Otitis media and sinusitis
Amoxicillin has replaced AMP for the treatment of these infections. Amoxicillin is commonly used to treat middle ear infections, which are usually caused by pneumococci and/or H. influenzae. Most authors advocate a 10- to 14-day course of chemotherapy for this disease (Bluestone, 1982; Feder, 1982; Kaleida et al., 1991). However, there is controversy as to whether antibiotics are necessary to treat most cases of otitis media, which is often self-limiting (Damoiseaux et al., 2000). In a randomized, double-blind, placebo-controlled trial of amoxicillin in children aged six months to five years of age, 92.8% of the children responded satisfactorily to amoxicillin compared with 84.2% of the children receiving placebo (Le Saux et al., 2005).
c. Urinary tract infections
Acute, uncomplicated urinary tract infections caused by E. coli and P. mirabilis usually respond to AMP if the infecting organism is sensitive. The drug is also useful for the treatment of E. faecalis infections. High concentrations of AMP are attained in urine, and treatment is sometimes successful despite the demonstrations of in vitro resistance of the causative organism.
Amoxicillin has largely replaced ampicillin for the treatment of urinary tract infections and is now widely used. However, the emergence of resistance among causative organisms has led to a decrease in its usefulness for this indication. In women with infections restricted to the bladder, a single amoxicillin dose of 3 g appeared to be just as effective as a 14-day course of 250 mg given three times a day (Fang et al., 1978; Savard-Fenton et al., 1982; Tolkoff-Rubin et al., 1984).
d. Respiratory tract infections
Pneumococcal lobar pneumonia responds to AMP–AMOX, but penicillin G is often as effective. AMP should not be used alone for life-threatening forms of pneumonia in which the bacteriologic etiology cannot be immediately determined, since it is ineffective against many of the other pathogens that can cause serious pneumonia.
Amoxicillin is recommended for the treatment of uncomplicated mild community-acquired pneumonia (MacFarlane and Boldy, 2004). If nonmethicillin-resistant S. aureus (MRSA) infection is suspected (e.g. associated with influenza), flucloxacillin should be added and, if atypical pathogens are a possibility, a macrolide or doxycycline should be given in addition to amoxicillin (Therapeutic guidelines, 2006).
e. Infective endocarditis
Similar to penicillin G, AMP–AMOX is given intravenously in combination with an aminoglycoside for the treatment of enterococcal endocarditis (see Chapter 1, Benzylpenicillin (Penicillin G)) (Elliott et al., 2004).
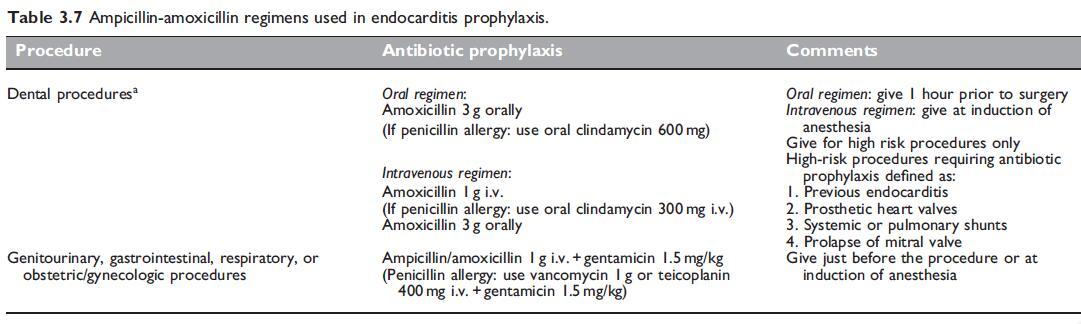
Amoxicillin has been recommended as a prophylactic agent for the prevention of endocarditis. Various regimens and indications are summarized in Table 3.7. Amoxicillin has been shown to reduce the incidence of bacteremia associated with dental procedures (Lockhart et al., 2004). As a consequence, amoxicillin in a single dose of 2 or 3 g, given 1 hour prior to surgery, has been recommended as preoperative prophylaxis for patients with heart valve lesions undergoing dental procedures (Dajani et al., 1997).
f. Neonatal septicemia
AMP or AMOX is used for the treatment of suspected neonatal septicemia, as they are active against group B streptococci, L. monocytogenes, E. faecalis, and P. mirabilis. In neonates, they are usually combined with either gentamicin or amikacin, to provide cover for AMP-resistant Gram-negative bacilli, such as E. coli, Klebsiella spp., and P. aeruginosa (Kaplan et al., 1974; McCracken and Eichenwald, 1974).
g. Dental infections
Amoxicillin is commonly used to treat intraoral infections. A combination of ampicillin and metronidazole proved effective in the treatment of periodontitis (Winkel et al., 2001). h. Group B streptococcal infections Women who have had group B streptococci cultured from urine during pregnancy should be given i.v. intrapartum prophylaxis with penicillin, ampicillin, or amoxicillin (MMWR, 2002).
i. Typhoid fever and other Salmonella infections
AMP is not as effective as chloramphenicol for the treatment of typhoid fever; defervescence is slower and some severe cases fail to respond to AMP, whereas response to chloramphenicol is uniformly good (Manriquez et al., 1965; Sanders, 1965; Snyder et al., 1976). In addition, positive stool cultures persist longer in AMP-treated patients than in those treated by chloramphenicol (Snyder et al., 1976). Chloramphenicol is also superior to AMP for the treatment of paratyphoid fever, even when relatively large doses of AMP (6 g/day in adults) are used (Sleet et al., 1964). Therefore, in most parts of the world, chloramphenicol was formerly preferred to AMP for the treatment of typhoid and paratyphoid fevers (Svenungsson, 1982).
j. Shigella infections
Shigella sonnei dysentery is frequently a self-limiting disease, for which rehydration is the most important measure. In contrast to S. enteritis, provided the strain is sensitive, AMP is of clinical benefit in shigellosis, both in milder cases treated as outpatients (Haltalin et al., 1972) and in severe S. flexneri infections in hospitalized children (Haltalin et al., 1967). The drug also reduces the duration of fecal excretion of shigellae (McCracken and Eichenwald, 1974). Children with shigellosis who are treated with ampicillin also absorb nutrients more efficiently from the bowel during the acute stage of the disease, than do untreated controls (Molla and Molla, 1991).
k. Gonorrhoea
Amoxicillin in a single dose of 3 g plus 1 g probenecid was widely recommended as a suitable ‘‘single-dose’’ regimen for the treatment of this disease. However, it is ineffective for the disease caused by penicillin G-resistant strains which are either intrinsically resistant or beta-lactamase producers (see Chapter 1, Benzylpenicillin (Penicillin G)). Such strains are now common in the USA, Australia, and many other countries. Intramuscular ceftriaxone is now most commonly recommended for the treatment of gonorrhoea (MMWR, 2007). In areas where penicillin G-resistant strains of gonococci are still uncommon, amoxicillin can be used with success.
l. Helicobacter pylori infection
Helicobacter pylori eradication reduces the recurrence of gastric and duodenal ulcers. Various regimes have been recommended for eradication, including a proton pump inhibitor such as omeprazole plus clarithromycin, and either amoxicillin or metronidazole (Bayerdorffer et al., 1999; Nagahara et al., 2004). Resistance to amoxicillin is rare, but resistance to metronidazole and clarythromycin is common. A combination of furazolidine with amoxicillin and omeprazole has been shown to be effective when resistance to metronidazole is common (Roghani et al., 2003).
m. Pertussis
AMP may be useful for the treatment and prevention of secondary pulmonary infection in this disease. The main factor in reducing the once large mortality in pertussis has probably been the use of antibiotics to prevent and treat secondary bronchopneumonia, particularly in young babies (Bennett, 1973). AMP, similar to AMOX and other antibiotics, does not shorten the clinical course of whooping cough. Erythromycin, oxytetracycline, and chloramphenicol eliminate B. pertussis within a few days (presumably rendering patients noninfectious), whereas AMP-treated patients have positive cultures for periods similar those of untreated patients (Bass et al., 1969). For this reason, erythromycin or another macrolide is generally regarded as the drug of choice for treatment of patients with pertussis (Bass, 1973; Zackrisson et al., 1983).
References
Acred P, Hunter PA, Mizen L, Rolinson GN (1971). Alpha-amino-phydroxybenzyl-
penicillin (BRL 2333), a new broad-spectrum semisynthetic
penicillin: In vivo evaluation. Antimicrob Agents Chemother 1970:
416.
ADRAC (Adverse Drug Reactions Advisory Committee 1978). Ampicillin and
amoxycillin – identical twins. Asut Presc 2: 81.
Afifi AM, Adnan M, El Garf AA (1976). Amoxycillin in treatment of typhoid
fever in patients with haematological contraindications to chloramphenicol.
Br Med J 2: 1033.
Albritton WL, Hammond G, Hoban S, Ronald AR (1977). Ampicillin-resistant
H. influenzae subdural empyema following successful treatment of apparently
ampicillin-sensitive H. influenzae meningitis. J Pediatr 90: 320.
Ali HM (1985). Reduced ampicillin bioavailability following oral
coadministration with chloroquine. J Antimicrob Chemother 15: 781.
Angkititrakul S, Chimvarin C, Chaita Tet al. (2005). Epidemiology of
antimicrobial resistance in Salmonella isolated from pork, chicken meat and
humans in Thailand. Southeast Asian J Trop Med Pub Hlth 36: 1510.
Anthonisen NR, Manfreda J, Warren CPW et al. (1987). Antibiotic therapy in
exacerbations of chronic obstructive pulmonary disease. Ann Intern Med
106: 196.
Anon (1995). Recommendations for preventing the spread of vancomycin
resistance. Recommendations of the Hospital Infection Control Practices
Advisory Committee (HICPAC). MMWR 44 (RR-12): 1.
Arndt KA, Jick H (1976). Rates of cutaneous reactions to drugs A report from
the Boston Collaborative Drug Surveillance Program. JAMA 235: 918.
Asensi V, Fierer J (1991). Synergistic effect of human lysozyme plus ampicillin or
beta-lysin on the killing of Listeria monocytogenes. J Infect Dis 163: 574.
You may like
Related articles And Qustion
Lastest Price from Amoxicillin manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 26787-78-0
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $5.00-0.50/KG2025-05-09
- CAS:
- 26787-78-0
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS