Tofacitinib Citrate: Mechanism of Action and Clinical Applications in Psoriasis
General Description
Tofacitinib citrate is the citrate form of tofacitinib, a highly potent, orally active Janus kinase (JAK) inhibitor approved for the treatment of moderate-to-severe rheumatoid arthritis (RA) with an inadequate response or intolerance to methotrexate. Tofacitinib is also indicated for the treatment of adult patients with active psoriatic arthritis, ankylosing spondylitis, moderate to severe ulcerative colitis, and children 2 years of age and older with active polyarticular juvenile idiopathic arthritis. It is also used to treat inflammatory skin conditions such as atopic dermatitis, psoriasis and plaque psoriasis.

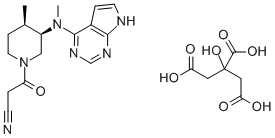
Figure 1. Tofacitinib citrate
Mechanism of Action
Psoriasis is characterised by the activation of several different types of immune cells that infiltrate the skin, stimulate the proliferation of keratinocytes and produce pro-inflammatory cytokines. Effective activation of immune cells requires the integration of external stimuli via their surface receptors. Attachment between the receptor and its ligand leads to activation of intracellular kinases. This enzyme phosphorylates downstream molecules, creating a cascade reaction that leads to the transcription of genes encoding effector molecules. Therefore, inhibition of JAK also suppresses immune cell activation and inflammatory responses in psoriasis.
Tofacitinib inhibits the phosphorylation of JAK1 and JAK3, IL-6-driven phosphorylation of STAT1 and STAT3, and STAT5. Tofacitinib is a JAK inhibitor, preferentially inhibiting JAK1 and JAK3, followed by JAK2, with minimal effect on TYK2. Its primary targets are dendritic cells, CD4(+) T cells (e.g., Th1 and Th17), and activated B cells, resulting in multicytokine targeting.Tofacitinib inhibits type I interferon-mediated signalling, which inhibits the antigen-presenting and T-cell-stimulating capacity of dendritic cells. It limits the production of IL-17A, IL-17F and IL-22, the expression of IL-23R and the differentiation of Th1 cells. Since IL-23 is one of the key cytokines affecting the development and function of Th17 cells, by inhibiting the expression of IL-23 receptor, the differentiation of Th17 cells is also reduced.
Clinical Applications in Psoriasis
Efficacy in Moderate-to-Severe Psoriasis
Tofacitinib citrate has demonstrated significant clinical efficacy in treating moderate-to-severe chronic plaque psoriasis, as revealed through multiple placebo-controlled and randomized clinical trials. In two pivotal Phase III studies, patients receiving a dosage of tofacitinib citrate at 10 mg twice daily (bid) showed considerable improvements compared to those on 5 mg bid and placebo. The results indicated that the rates of achieving a 75% reduction in the Psoriasis Area and Severity Index (PASI 75) were 39.9% and 59.2% for the 5 mg and 10 mg doses, respectively, after 16 weeks in the Opt Pivotal 1 study. Similarly, in the Opt Pivotal 2 study, the PASI 75 response rates were 46.0% for the 5 mg group and 59.6% for the 10 mg group. These findings underscore the efficacy of tofacitinib citrate, particularly at the higher dosage, in significantly reducing the severity of psoriasis lesions. 2
Long-term Effects and Treatment Duration
Further clinical investigations have explored the outcomes of tofacitinib citrate after treatment cessation. After a 24-week treatment period, 33.5% of patients on the 5 mg dose and 55.2% on the 10 mg dose achieved both PASI 75 and PGA responses. Notably, patients who continued on tofacitinib citrate maintained their treatment responses significantly better compared to those who received a placebo after cessation of treatment. Among those who experienced a relapse, up to 60% were able to regain a satisfactory response upon reinitiating therapy with tofacitinib citrate. This highlights the sustained effectiveness of tofacitinib citrate, particularly at the 10 mg bid dosage, for managing long-term psoriasis symptoms and achieving consistent patient outcomes. However, questions about safety risks above the 5 mg bid dose remain unresolved.2
References:
[1] XINGZHONG JIN. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis.[J]. Annals of the Rheumatic Diseases, 2015, 74 4. DOI:10.1136/annrheumdis-2013-204494.[2] V. DI LERNIA F B. Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis[J]. Drug Design, Development and Therapy, 2016, 10 1: 533-539. DOI:10.2147/DDDT.S82599.
[3] V. DI LERNIA F B. Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis[J]. Drug Design, Development and Therapy, 2016, 10 1: 533-539. DOI:10.2147/DDDT.S82599.
[4] SRIVIDYA ATMAKURI . Topical delivery of tofacitinib citrate loaded novel nanoemulgel for the management of 2,4-Dichlorodinitrobenzene induced atopic dermatitis in mice model[J]. Journal of Drug Delivery Science and Technology, 2023, 80. DOI:10.1016/j.jddst.2022.104145.
You may like
Related articles And Qustion
See also
Lastest Price from Tofacitinib citrate manufacturers

US $0.00/kg2025-11-28
- CAS:
- 540737-29-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $1.00/KG2025-10-14
- CAS:
- 540737-29-9
- Min. Order:
- 10000KG
- Purity:
- 99.99%
- Supply Ability:
- 20 TONS