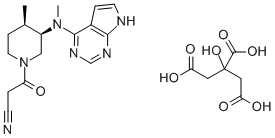
FDA-approved drug Tofacitinib citrate: Brand name,Indications and Side Effects
Tofacitinib citrate (XELJANZ) was the first FDA-approved pill of its kind (JAK inhibitor) that treats adults with moderate to severe rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe ulcerative colitis. It is also approved for patients ages 2 and older with active polyarticular course juvenile idiopathic arthritis.

Brand name
Xeljanz
Indications
Tofacitinib citrate is an oral drug used to treat adults with moderate to severe active rheumatoid arthritis (RA), active psoriatic arthritis, ankylosing spondylitis, moderate to severe ulcerative colitis, and children ages 2 and older with activepolyarticular juvenile idiopathic arthritis.
Side Effects
Most common adverse reactions of Tofacitinib citrate are:
Rheumatoid and Psoriatic Arthritis: Reported during the first 3 months in rheumatoid arthritis controlled clinical trials and occurring in ≥2% of patients treated with XELJANZ monotherapy or in combination with DMARDs: upper respiratory tract infection, nasopharyngitis, diarrhea, and headache.
Ulcerative Colitis: Reported in ≥5% of patients treated with either 5 mg or 10 mg twice daily of XELJANZ and ≥1% greater than reported in patients receiving placebo in either the induction or maintenance clinical trials: nasopharyngitis, elevated cholesterol levels, headache, upper respiratory tract infection, increased blood creatine phosphokinase, rash, diarrhea, and herpes zoster.
Mechanism of Action
Tofacitinib citrate is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of hematopoiesis and immune cell function. Within the signaling pathway, JAKs phosphorylate and activate Signal Transducers and Activators of Transcription (STATs) which modulate intracellular activity including gene expression. Tofacitinib modulates the signaling pathway at the point of JAKs, preventing the phosphorylation and activation of STATs. JAK enzymes transmit cytokine signaling through pairing of JAKs (e.g., JAK1/JAK3, JAK1/JAK2, JAK1/TyK2, JAK2/JAK2). Tofacitinib inhibited the in vitro activities of JAK1/JAK2, JAK1/JAK3, and JAK2/JAK2 combinations with IC50 of 406, 56, and 1377 nM, respectively.
Pharmacodynamic effects
In patients with RA, treatment up to 6 months with tofacitinib citrate was associated with dose-dependent reductions of circulating CD16/56+ natural killer (NK) cells, with estimated maximum reductions occurring at approximately 8-10 weeks after initiation of therapy. These changes generally resolved within 2-6 weeks after discontinuation of treatment. Treatment with tofacitinib was associated with dose-dependent increases in B cell counts. Changes in circulating T-lymphocyte counts and T-lymphocyte subsets (CD3+, CD4+ and CD8+) were small and inconsistent.
Following long-term treatment (median duration of tofacitinib treatment of approximately 5 years), CD4+ and CD8+ counts showed median reductions of 28% and 27%, respectively, from baseline. In contrast to the observed decrease after short-term dosing, CD16/56+ natural killer cell counts showed a median increase of 73% from baseline. CD19+ B cell counts showed no further increases after long-term tofacitinib treatment. All these lymphocyte subset changes returned toward baseline after temporary discontinuation of treatment. There was no evidence of a relationship between serious or opportunistic infections or herpes zoster and lymphocyte subset counts.
Related articles And Qustion
See also
Lastest Price from Tofacitinib citrate manufacturers

US $0.00/kg2025-11-28
- CAS:
- 540737-29-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $1.00/KG2025-10-14
- CAS:
- 540737-29-9
- Min. Order:
- 10000KG
- Purity:
- 99.99%
- Supply Ability:
- 20 TONS