Tirabrutinib: Synthesis and Introduction
Synthesis of Tirabrutinib
Tirabrutinib is synthesised using 4-phenoxyaniline as a raw material by chemical reaction in the presence of a phase transfer catalyst (TBAB). The specific synthesis steps are as follows:
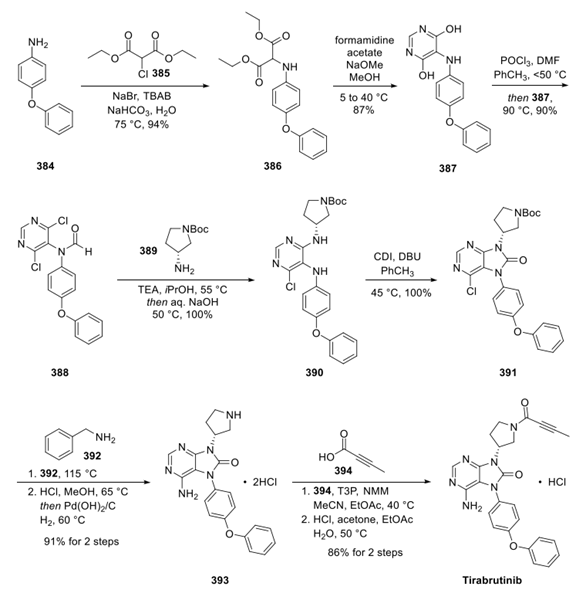
This kilogram-scale synthesis commenced with 4-phenoxyaniline 384, which reacted with chloromalonate 385 in aqueous media in the presence of a phase transfer catalyst (TBAB) to furnish aminomalonate 386. Condensation with formamidine acetate produced pyrimidine 387 in 87% yield. Pyrimidine diol 387 was converted to the corresponding dichloroaryl system 388 upon subjection to phosphorus oxychloride in DMF. Fortuitously, these conditions also resulted in N-formylation of the aniline nitrogen, presumably through a Vilsmeier−Haack type mechanism. Substitution with 389 required careful control of stoichiometry with the authors reporting using a slight excess of 1 equiv of the nucleophile in order to achieve sufficient conversions. A subsequent workup with sodium hydroxide removed the formyl group to deliver diamine intermediate 390. The CDI-mediated azabenzimidazolone establishment took place before a second SNAr reaction with benzyl amine 392, and acidic deprotection generated diamine 393 as a dihydrochloride salt. Finally, T3P-mediated amidation of pyrrolidine 393 with butynoic acid (394), and the subsequent salt formation furnished tirabrutinib as the hydrochloride salt.
Introduction of Tirabrutinib
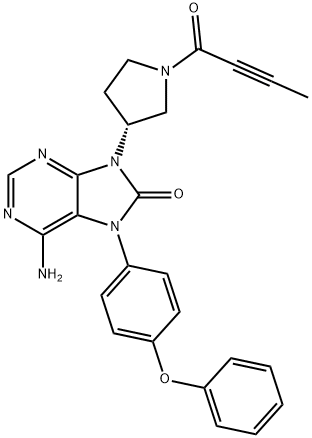
Tirabrutinib is an orally active Bruton's tyrosine kinase (BTK) inhibitor. The drug was approved for the treatment of recurrent or refractory primary central nervous system lymphoma in Japan in March 2020 with a recommended dosage of 480 mg once daily on an empty stomach. Tirabrutinib is under regulatory review for additional indications such as Waldenstrom's macroglobulinemia and lymphoplasmacytic lymphoma in Japan. BTK is an essential effector for B cell development and plays a critical role in lymphoma genesis. Tirabrutinib selectively and covalently binds to the cysteine-481 residue of BTK thereby inhibiting B cell-related malignancies and autoimmune diseases.
Tirabrutinib is the fourth BTK inhibitor to be approved, joining ibrutinib (first approved in 2013), acalabrutinib (2017), and zanubrutinib (2019), the latter three each approved by the USFDA. Compared to ibrutinib, tirabrutinib demonstrates greater selectivity against EGFR, ITK, and Tec family kinases and, hence, exhibits fewer off-target toxicities.
You may like
Related articles And Qustion
See also
Lastest Price from Tirabrutinib manufacturers

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10kg

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG