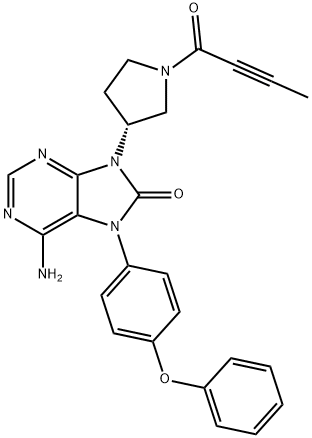
Description
Btk Kinase inhibitor is highly potent and selective orally administered, small molecule, and also named tirabrutinib, Brutons tyrosine kinase (BTK) inhibitor being developed by Ono pharmaceutical and its licensee Gilead Sciences for the treatment of autoimmune disorders and haematological malignancies.
Biological Functions
In March 2020, tirabrutinib was approved in Japan to treat primary central nervous system lymphoma (PCNSL). Tirabrutinib shows excellent selectivity against all kinases with cysteines in the ATP-binding site corresponding to Cys481 in BTK with the exception of BMX, TXK, and TEC? However, it displays weaker BTK inhibition than ibrutinib (IC50 = 6.8 nM vs. 0.47 nM).
General Description
Class: non-receptor tyrosine kinase
Treatment: PCNSL
Elimination half-life = 6.5–8 h
Protein binding = 91%
Mechanism of action
The selective inhibition of cell growth by Btk Kinase inhibitor was due to blocking of BTK-mediated signaling through AKT and cellular protein kinase D. It can inhibit autophosphorylation of the BTK at the Tyr223 position through the ERK, AKT and PKD signaling pathways.
Side effects
Adverse effects of the Btk Kinase inhibitor that occurred in some patients were rash, vomiting, neutropenia, arthralgia, and malaise, and drug-related Grade 3–4 Adverse effects were neutropenia, leukopenia, anemia, hypophosphatemia, PT-INR increased, pneumonitis, and acute myeloid leukemia. in prior Japanese studies, rash, hematologic adverse effects, erythema multiforme, and constipation were frequent adverse effects in a Phase I/II study of some patients with PCNSL, and rash, hematologic adverse effects, and stomatitis were the most common adverse effects in a Phase II study of some patients with WM.