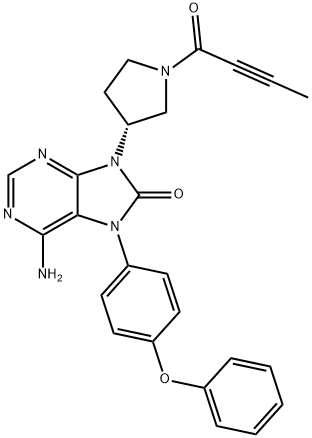
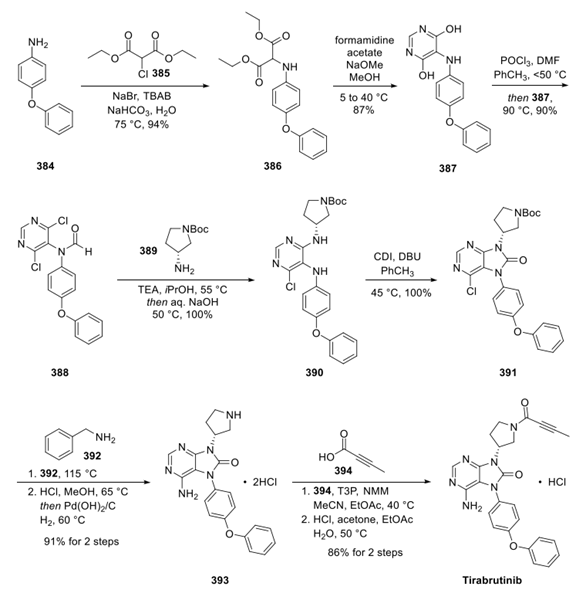
Btk Kinase Inhibitor: Pharmacodynamics, Pharmacokinetics and Adverse Events
Description
Btk Kinase Inhibitor, a potent and selective Bruton's tyrosine kinase (BTK) inhibitor, exerts its pharmacodynamics through irreversible covalent binding to BTK Cys-481. It demonstrates remarkable selectivity for BTK and TEC, inhibiting proliferation and inducing apoptosis in B cell lymphoma cell lines.

Pharmacodynamics
BTK plays a crucial role in B cell development as it is required for transmitting signals from the pre-B cell receptor that forms after successful immunoglobulin heavy chain rearrangement. It also has a role in mast cell activation through the high-affinity IgE receptor.
BTK contains a PH domain that binds phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 binding induces BTK to phosphorylate phospholipase C (PLC), which in turn hydrolyzes PIP2, a phosphatidylinositol, into two second messengers, inositol triphosphate (IP3) and diacylglycerol (DAG), which then go on to modulate the activity of downstream proteins during B-cell signalling.1
Pharmacokinetics
Btk Kinase Inhibitor's pharmacokinetics involve rapid absorption, with peak plasma concentrations achieved around 2.87 hours post-administration. Btk Kinase Inhibitor exhibits high plasma protein binding (92%) and is predominantly metabolized by CYP3A4 enzymes. Metabolites M33 and M12, along with the unchanged parent drug, account for the majority of plasma radioactivity post-administration. Elimination occurs primarily via feces (52.2%) and urine (42.1%), with a mean elimination half-life of approximately 3.55 hours. Coadministration with CYP3A inhibitors or inducers may alter Btk Kinase Inhibitor exposure, and concurrent use with anticoagulant/antiplatelet agents may increase the risk of bleeding. 2
Adverse Events
Possible BTK inhibitor side effects include fatigue, rash, diarrhea, bleeding and bruising. Some patients experience neutropenia, which is caused when a patient’s white blood cell count drops. That means they’re at a higher risk of infection. Patients taking ibrutinib can experience an irregular heartbeat and hypertension.
1. Lowe J, Joseph RE, Andreotti AH (1 January 2022). "Conformational switches that control the TEC kinase - PLCγ signaling axis". Journal of Structural Biology. 6: 100061.
2.Ono Pharmaceutical Co. VELEXBRUⓇ (Tirabrutinib) 80 mg tablets: Japanese prescribing information. 2020.
References:
[1] JACQUES LOWE A H A Raji E Joseph. Conformational switches that control the TEC kinase – PLCγ signaling axis[J]. Journal of Structural Biology: X, 2022, 6. DOI:10.1016/j.yjsbx.2022.100061.Related articles And Qustion
Lastest Price from Tirabrutinib manufacturers

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10kg

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG