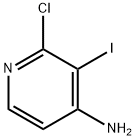
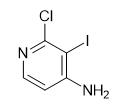
Exploring the Versatile World of 2-Chloro-3-iodopyridin-4-amine: Insights into Its Structure, Mechanism, and Multifaceted Applications
Introduction
2-Chloro-3-iodopyridin-4-amine is a compound that has recently garnered significant attention in the field of chemistry, particularly in pharmaceutical research and development. Its unique structure, characterized by the presence of both chlorine and iodine atoms on the pyridine ring, renders it a valuable building block in organic synthesis. The compound's ability to act as a versatile intermediate has led to its use in the synthesis of a wide range of pharmaceuticals, agrochemicals, and advanced materials. Recent developments have further highlighted its potential in drug discovery, making it a topic of considerable interest among chemists and researchers in related fields[1].

Figure 1 Characteristics of 2-Chloro-3-iodopyridin-4-amine
Structure and Properties
2-Chloro-3-iodopyridin-4-amine exhibits a fascinating chemical structure that significantly contributes to its broad utility in various applications. The molecule consists of a pyridine ring, a basic structure in organic chemistry known for its aromaticity and electron-rich nature. This ring is substituted at the second, third, and fourth positions with a chlorine atom, an iodine atom, and an amine group, respectively. The presence of both electronegative chlorine and iodine atoms imparts unique electronic properties to the molecule, affecting its reactivity and interaction with other compounds.
The physical properties of 2-Chloro-3-iodopyridin-4-amine are equally noteworthy. It typically appears as a crystalline solid under standard conditions. The melting point, solubility, and other physical characteristics are determined by its molecular structure. The polar nature of the compound, owing to its halogen atoms and amine group, influences its solubility in various solvents, an important consideration in chemical synthesis and applications.
From a chemical standpoint, the compound exhibits typical reactivity patterns of halogenated aromatics and amines. Its reactivity is further influenced by the presence of both electron-withdrawing and electron-donating groups, leading to a unique set of reactions and pathways that it can undergo. This reactivity profile is crucial in its applications, especially in organic synthesis, where it acts as an intermediate or a reactant.
Mechanism of Action
The mechanism of action of 2-Chloro-3-iodopyridin-4-amine in chemical reactions is a cornerstone of its utility in various fields. This compound plays a critical role primarily due to the presence of both a reactive amine group and halogen atoms, which offer multiple pathways for chemical interactions and transformations.
At its core, 2-Chloro-3-iodopyridin-4-amine acts as a versatile nucleophile and electrophile, thanks to its amine group and halogen atoms, respectively. The amine group, being a lone pair donor, can engage in nucleophilic substitution reactions, making it a key participant in the formation of carbon-nitrogen bonds. This reactivity is particularly crucial in the synthesis of heterocyclic compounds, a class of compounds widely used in pharmaceuticals.
The iodine and chlorine atoms, on the other hand, make the compound an excellent candidate for electrophilic substitution reactions. The iodine atom, being a good leaving group, facilitates various substitution reactions, where it can be replaced by other functional groups, leading to the formation of new compounds. This characteristic is especially valuable in cross-coupling reactions, a widely used methodology in the synthesis of complex organic molecules.
Moreover, the electronic interplay between the chlorine, iodine, and amine groups on the pyridine ring creates a unique electronic environment that can influence reaction pathways and outcomes. This aspect is particularly important in catalysis, where 2-chloro-3-iodopyridin-4-amine can act as a ligand, influencing the reactivity of metal catalysts in various chemical transformations.
Applications
The diverse applications of 2-Chloro-3-iodopyridin-4-amine span across various sectors, most notably in pharmaceuticals, agrochemicals, and material science. Its unique chemical structure and reactivity profile make it an invaluable tool in these fields.
Pharmaceuticals
In the pharmaceutical industry, 2-chloro-3-iodopyridin-4-amine is a critical intermediate in the synthesis of a wide range of therapeutic compounds. Its ability to undergo a variety of chemical reactions makes it a key component in the development of new drugs, particularly those involving complex heterocyclic structures. For example, it's used in the synthesis of kinase inhibitors, which play a significant role in cancer treatment. Its versatility also extends to the development of neurological drugs, where it forms the core structure for compounds targeting various neurological pathways.
Agrochemicals
In the realm of agrochemistry, this compound finds applications in the synthesis of herbicides and pesticides. The effectiveness of these agrochemicals often hinges on the ability to interact with specific biological pathways in pests or weeds, and 2-Chloro-3-iodopyridin-4-amine provides a foundational structure for these interactions. Its reactivity allows for the creation of compounds that are specific in their action, reducing collateral environmental impact.
Material Science
Beyond life sciences, 2-Chloro-3-iodopyridin-4-amine is instrumental in material science, particularly in the synthesis of novel organic materials. Its inclusion in polymers and other advanced materials can impart desirable properties like increased durability, improved electrical conductivity, and enhanced chemical resistance[2].
Storage and Handling
Proper storage and handling of 2-Chloro-3-iodopyridin-4-amine are crucial for maintaining its integrity and ensuring safety in the workplace. The compound, like many halogenated organics, requires specific conditions to remain stable and to minimize any risks associated with its handling.
Storage Conditions
2-Chloro-3-iodopyridin-4-amine should be stored in a cool, dry, and well-ventilated area, away from direct sunlight and extreme temperatures. The compound is best kept in airtight containers made of materials that do not react with halogenated compounds. Moisture control is essential, as exposure to water or humidity can lead to degradation or unwanted reactions. It's also important to store it separately from incompatible substances, such as strong oxidizing agents, to prevent hazardous reactions.
Handling Precautions
When handling 2-Chloro-3-iodopyridin-4-amine, appropriate personal protective equipment (PPE) is essential. This includes gloves, safety goggles, and lab coats. Given its potential reactivity and the presence of iodine and chlorine, care should be taken to avoid inhalation, ingestion, or skin contact. Working in a fume hood is advisable to ensure adequate ventilation and to protect against any fumes or accidental exposure.
References
[1]Guillemont J, Benjahad A, Oumouch S, et al. Synthesis and Biological Evaluation of C-5 Methyl Substituted 4-Arylthio and 4-Aryloxy-3-Iodopyridin-2 (1 H)-one Type Anti-HIV Agents[J]. Journal of medicinal chemistry, 2009, 52(23): 7473-7487.
[2]Balfour M N, Franco C H, Moraes C B, et al. Synthesis and trypanocidal activity of a library of 4-substituted 2-(1H-pyrrolo [3, 2-c] pyridine-2-yl) propan-2-ols[J]. European Journal of Medicinal Chemistry, 2017, 128: 202-212.
Related articles And Qustion
Lastest Price from 2-CHLORO-3-IODOPYRIDIN-4-AMINE manufacturers
US $200.00-1.00/KG2024-03-25
- CAS:
- 909036-46-0
- Min. Order:
- 1KG
- Purity:
- 99%, 99.5% Sublimated
- Supply Ability:
- g-kg-tons, free sample is available