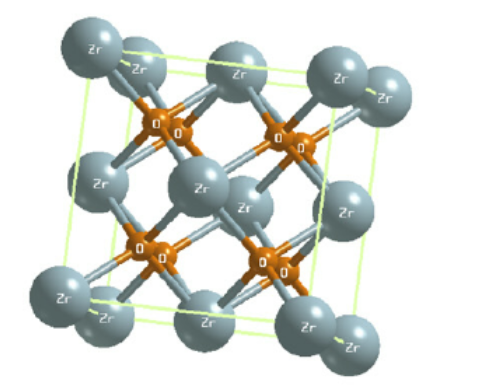
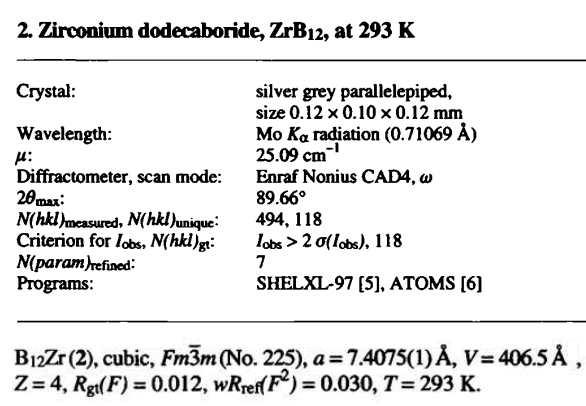
The zirconium C boron system: zirconium dodecaboride
Description
Metal dodecaborides (MB12) materials are the subject of much scientific research due to their interesting combination of excellent properties such as high melting points, hardness, and thermal and chemical stability. The clathrate-like rigid network composed of boron atoms makes them serve as a paradigm for other cluster compounds such as fullerides or higher borides[1].
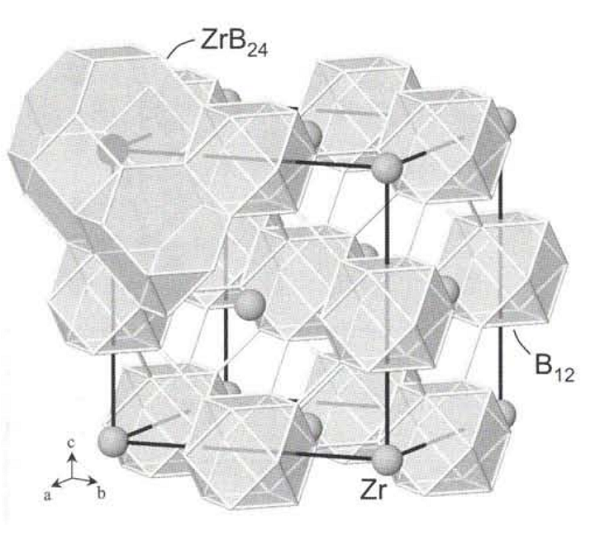
Among the different alloys of the zirconium C boron system, zirconium dodecaboride (ZrB12) is known as a material possessing rather high abrasion properties. ZrB12 may be used especially effectively in polishing titanium and titanium alloys. Zirconium dodecaboride is produced by arc melting of a charge of the prescribed composition. The ingot that has been formed in a crystallizer is crushed in order to obtain abrasive powders of different grain sizes. The abrasive disks that are produced from these powders have demonstrated not only a high degree of efficiency in the treatment of the titanium alloy VT-1 comparable with that of diamond disks of the same grain size but also yield a significant decrease in the surface roughness of the worked part.
Structure
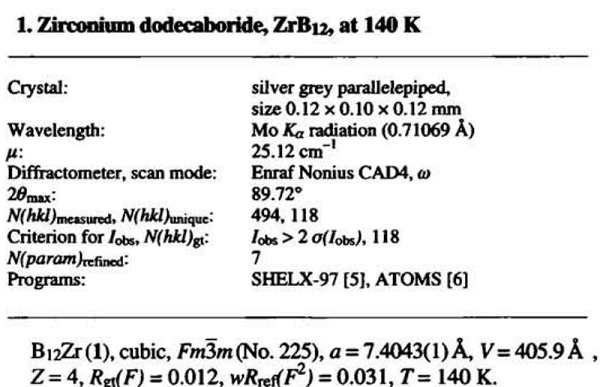
ZrB 12 adopts the UB12 type of structure. The boron atoms form one type of Β12 cuboctahedral unit. The interconnection of the cuboctahedra gives rise to large truncated octahedra centered on Zr atoms and truncated tetrahedra. The bond lengths between boron atoms are observed in the 1.68 À < <¡bb < 1.78 À. The shorter is an inter-cuboctahedral one, and the longer is an intra-cuboctahedral one[2].
Zirconium Dodecaboride-based cutting material.
Rugged cutting elements of ZrB12 have been fabricated using arc (type I) or induction (type II) melting. Arc melting of compacts from a mixture of powders of zirconium and boron of stoichiometric composition has been produced in a standard laboratory-scale furnace equipped with a copper water-cooled crystallizer and non-consumable tungsten electrode in a purified argon medium. From the melting point (2082EC) to a temperature of around 1500EC, the cooling rate amounted to roughly 400 deg/sec, which was then gradually reduced to 150 deg/sec until the ingot completely cooled down. Directed crystallization of the melt was achieved in a Kristall III induction plant following the formation of a fusion zone at a temperature corresponding to the phase diagram of Zr − B. Preliminarily sintered porous rod billets were used as the initial material for directed crystallization. The charge was in the form of a mixture of zirconium powder and powder of finely crystallized boron. The boron was taken in excess with respect to stoichiometry. The charge components were carefully mixed in an ultrasonic vibrator cavitation bath. Rods were compacted from the obtained mixture in a steel die-pressure cast mold and sintered in a vacuum at 1600EC [3].
Following arc melting, the type I cutting element is a typical polycrystalline material with interlayers of zirconium diboride between the grains of the primary phase; these latter sharply reduce the thermal conductivity of the material and, consequently, reduce heat removal from the cutting edge. The type II element is a single-phase material since, in the course of zone melting, the material is purified of impurities, thus increasing the degree of perfection of the structure of the cutting element as well as increasing its thermal conductivity and, as a consequence, improving heat removal from the cutting zone. Thus, a tool equipped with materials based on zirconium dodecaboride (ZrB12) fabricated by melting as a cutting element makes it possible to machine titanium and titanium alloys at the level of precision turning.
References
[1] Bangcheng Ai . “Theoretical elastic stiffness and thermodynamic properties of zirconium dodecaboride from first principles calculation.” Computational Materials Science 82 (2014): Pages 37-44.
[2] A. Leithe-Jasper, T. Tanaka, A. Sato. “Refinement of the crystal structure of zirconium dodecaboride, ZrB12, at 140 Κ and 293 Κ.” Zeitschrift Fur Kristallographie-new Crystal Structures 497 1 (2002): 319–320.
[3] Y. Paderno. “Zirconium Dodecaboride-based cutting material.” Powder Metallurgy and Metal Ceramics 17 1 (2004): 546–548.