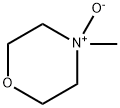
The Versatile Solvent: 4-Methylmorpholine N-oxide
Introduction to 4-Methylmorpholine N-oxide
4-Methylmorpholine N-oxide is an organic compound.is a special solvent with excellent solubility for cellulose. It exists as a crystalline solid or liquid at room temperature, is non-toxic, weakly alkaline, and highly hygroscopic. It is a colorless or slightly yellow crystalline powder that is soluble in various organic solvents such as water, ethanol, and ether solvents.
This compound has a wide range of applications. It is commonly used as an oxidant to oxidize electrophilic reagents into alkaline reagents in organic synthesis reactions, thereby achieving various organic conversion reactions, such as the oxidation of alcohols to aldehydes or ketones, and the oxidation of amines to aldehydes or ketones. NMO can also be used as a desulfurizer, impurity remover, and redox catalyst, as well as for the dissolution and decolorization of cellulose.
The common method for preparing 4-methylmorpholine N-oxide is to react N-methylmorpholine with ammonium persulfate or sodium persulfate. The reaction is usually carried out at room temperature and often under alkaline conditions to produce the desired oxide. After the reaction is complete, the desired product can be obtained through conventional separation and purification methods. Next, I will further introduce some important information about this product for your reference and reference.

Figure 1 Characteristics of 4-Methylmorpholine N-oxide
Preparation Method
Method 1: Add 1.09 ml (10 mmol) of NMM solution in 10 ml of water to a 25 ml round-bottom flask. Pass CO2 gas through the solution at 1 atmosphere pressure for 10 minutes. Stir the reaction mixture and continue passing CO2 for 30 minutes. After the reaction is complete, remove the water by rotary evaporation to obtain the crude amine oxide product. Purify the crude product by column chromatography (silica gel, 100-200 mesh; MeOH/CH2Cl2=10:90) to obtain the titled compound, 4-Methylmorpholine N-oxide.
Method 2: In the presence of benzonitrile, add 0.22 ml (2 mmol) of N-methyl morpholine, 10 mg of catalyst B, and benzonitrile to a two-necked flask containing 10 ml of methanol. Dropwise add 0.66 ml (6 mmol) of 30% hydrogen peroxide solution for 15 minutes. Raise the temperature to 65°C and continue the reaction for 15 minutes. After the reaction is complete (followed by TLC), filter out the catalyst and wash it with methanol. Add a small amount of manganese dioxide to the filtrate to decompose the unreacted hydrogen peroxide. Filter the reaction mixture to remove solid MnO2 and concentrate the product under reduced pressure. Purify the resulting product, 4-methylmorpholine N-oxide, by column chromatography.
Uses
4-Methylmorpholine N-Oxide is a metabolite of Morpholine (M723725). N-methylmorpholine N-Oxide is commonly used to dissolve cellulose as well as in the dissolution of of scleroproteins.
4-Methylmorpholine N-oxide acts as a non-metallic catalyst for the cyanosilylation of ketones. It is also employed as a co-oxidant for Sharpless asymmetric dihydroxylation in ionic liquids. It serves as a solvent in the Lyocell process to produce Tencel fiber. Further, it is used in the preparation of aldehydes from primary alcohols in the presence of tetrapropylammonium perruthenate.
Non-metallic catalyst for the cyanosilylation of ketones. Co-oxidant for Sharpless asymmetric dihydroxylation in ionic liquids.
Purification Methods
When the oxide is dried for 2-3 hours at a high vacuum, it dehydrates. Add MeOH to the oxide and distill off the solvent under vacuum until the temperature is ca 95o. Then add Me2CO at reflux and cool to 20o. The crystals are filtered off, washed with Me2CO, and dried.
Safety information of 4-methyl morpholine N-oxide
4-Methylmorpholine N-oxide is an irritant compound that has a stimulating effect on the skin and eyes. Appropriate protective equipment such as gloves and goggles should be worn during operation, and good ventilation should be ensured. Long-term exposure to this substance should be avoided. If accidentally in contact with skin or eyes, rinse immediately with plenty of water.
![Article illustration]() Reference
Reference
[1] Rowley J R, Gabarayeva N I, Skvarla J J, et al. The Effect of 4-Methylmorpholine N-oxide Monohydrate (MMNO⊙ H~ 2O) on Pollen and Spore Exines[J]. TAIWANIA-TAIPEI-, 2001, 46(3): 246-273.
[2] Fernandes L C, Matos J R, Zinner L B, et al. Crystal structures, spectroscopic, TG and DSC studies of lanthanide picrate complexes with 4-methylmorpholine N-oxide (MMNO)[J]. Polyhedron, 2000, 19(22-23): 2313-2318.
References:
[1] ROWLEY J, GABARAYEVA N, SKVARLA J, et al. The Effect of 4-Methylmorpholine N-oxide Monohydrate (MMNO?H2O) on Pollen and Spore Exines[C]. 2001. DOI:10.6165/TAI.2001.46(3).246.[2] L. C. FERNANDES. Crystal structures, spectroscopic, TG and DSC studies of lanthanide picrate complexes with 4-methylmorpholine N-oxide (MMNO)[J]. Polyhedron, 2000, 19 1: 2313-2318. DOI:10.1016/S0277-5387(00)00494-0.
You may like
Lastest Price from 4-Methylmorpholine N-oxide manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 7529-22-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/KG2025-04-15
- CAS:
- 7529-22-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg