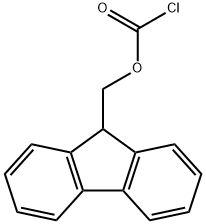
Fmoc-Chloride: A Cornerstone Reagent in Peptide Synthesis and Organic Chemistry
Introduction
Fmoc-Chloride (9-fluorenylmethyloxycarbonyl chloride) is a widely utilized chemical reagent in the field of organic chemistry, particularly in peptide synthesis. Its ability to protect amino groups during synthesis reactions has made it indispensable in the development of complex peptides and proteins. The Fmoc protecting group, combined with its ease of removal under mild conditions, offers a balance between stability and reactivity, making Fmoc-Chloride a preferred choice among chemists for amino acid protection.

Figure 1 Characteristics of Fmoc-Chloride
Properties
Fmoc-Chloride is an organic compound with the molecular formula C15H11ClO2. It is characterized by its pale yellow to brown crystalline appearance and a molecular weight of approximately 258.70 g/mol. The compound is relatively stable under normal laboratory conditions but can be sensitive to moisture, necessitating careful handling and storage.
Chemically, Fmoc-Chloride is known for its reactivity, especially towards nucleophiles such as amines. This reactivity is the cornerstone of its application in protecting amino groups in peptide synthesis. When an amino group reacts with Fmoc-Chloride, it forms a carbamate linkage, effectively "masking" the amino group from subsequent reactions until the Fmoc group is intentionally removed.
One of the significant advantages of Fmoc-Chloride is its ability to be cleaved under mildly basic conditions, typically using piperidine in DMF (dimethylformamide). This mild deprotection condition is particularly advantageous as it reduces the risk of damaging sensitive peptide bonds or other functional groups within the molecule. Furthermore, the removal of the Fmoc group generates a by-product, fluorenyl methanol, which is easily identifiable by UV spectrometry, providing a convenient monitoring method during synthesis.
Composition
The chemical structure of Fmoc-Chloride is based on the fluorenylmethyloxycarbonyl (Fmoc) group, which is attached to a chloride. The fluorenyl group contributes to the stability and reactivity of the molecule, while the chloride atom is crucial for the formation of the reactive intermediate that allows for the efficient protection of amino groups.
Fmoc-Chloride is typically synthesized from fluorenyl methanol, which undergoes a reaction with phosgene or its synthetic equivalents, such as triphosgene. The resulting Fmoc-Cl compound retains the aromatic fluorenyl group, which is crucial for its stability and ability to absorb UV light, making it detectable in various analytical methods.
The purity of Fmoc-Chloride is critical for its effectiveness in peptide synthesis. Impurities can lead to incomplete reactions or undesired side products, which can complicate the synthesis process. Therefore, commercially available Fmoc-Chloride is usually of high purity, often exceeding 98%, and is tested to ensure it meets stringent quality standards.
Applications
Fmoc-chloride is predominantly used in the field of peptide synthesis, where it serves as a protecting group for amino acids. The Fmoc group is attached to the amino group of an amino acid, preventing it from reacting during subsequent steps of peptide bond formation. This selective protection is crucial for the stepwise construction of peptides, particularly in solid-phase peptide synthesis (SPPS).
In SPPS, Fmoc-protected amino acids are sequentially added to a growing peptide chain anchored to a solid resin. After each amino acid is added, the Fmoc group is removed using a mild base, typically piperidine, to expose the amino group for the next coupling reaction. This process is repeated until the desired peptide sequence is achieved. The Fmoc strategy, as it is known, is favored for its compatibility with automated synthesis techniques and its ability to produce peptides with high purity and yield.
Beyond peptide synthesis, Fmoc-Chloride is also employed in the protection of other functional groups in organic synthesis, particularly where mild deprotection conditions are required. It is used in the synthesis of complex organic molecules, including pharmaceuticals and biologically active compounds, where the protection of specific functional groups is necessary to achieve the desired reaction outcomes.
Storage and Handling
Due to its sensitivity to moisture, Fmoc-Chloride must be stored under anhydrous conditions, typically in a tightly sealed container, in a cool and dry environment. Exposure to moisture can lead to hydrolysis, resulting in the formation of Fmoc-OH (9-fluorenyl methanol), which would render the reagent ineffective for its intended purpose.
The ideal storage temperature for Fmoc-Chloride is below 25°C, and it should be kept away from sources of heat and direct sunlight. In a laboratory setting, Fmoc-Chloride is often stored in a desiccator or a dry box, sometimes under an inert atmosphere such as nitrogen or argon, to further minimize the risk of moisture exposure.
When handling Fmoc-Chloride, appropriate personal protective equipment (PPE) should be worn, including gloves and eye protection, to prevent direct contact with the skin and eyes. Inhalation of Fmoc-Chloride dust or vapors should be avoided, and the use of a fume hood is recommended during its handling to ensure adequate ventilation.
Spills or accidental exposure to Fmoc-Chloride should be managed promptly by following standard laboratory safety protocols. Contaminated surfaces should be cleaned thoroughly, and any waste containing Fmoc-Chloride should be disposed of following local environmental regulations.
Conclusion
Fmoc-Chloride is a critical reagent in the field of organic chemistry, particularly in peptide synthesis. Its unique properties, including its stability, reactivity, and ease of removal, make it an invaluable tool for chemists. The compound's ability to protect amino groups under mild conditions has revolutionized peptide synthesis, enabling the production of complex peptides and proteins with high precision.
The careful handling and storage of Fmoc-Chloride are essential to maintaining its reactivity and ensuring successful outcomes in synthetic applications. As research in the field of organic chemistry and peptide synthesis continues to advance, Fmoc-Chloride will likely remain a cornerstone reagent, facilitating the development of new and innovative molecules in both academic and industrial settings.
[1] Behrendt R, White P, Offer J. Advances in Fmoc solid‐phase peptide synthesis[J]. Journal of Peptide Science, 2016, 22(1): 4-27.
[2] Geilfus C M. Chloride: from nutrient to toxicant[J]. Plant and Cell Physiology, 2018, 59(5): 877-886.
References:
[1] RAYMOND BEHRENDT J O Peter White. Advances in Fmoc solid-phase peptide synthesis[J]. Journal of Peptide Science, 2016, 22 1: i-iii, 4-66. DOI:10.1002/psc.2836.[2] GEILFUS C M. Chloride: from Nutrient to Toxicant.[J]. Plant and Cell Physiology, 2018, 59 5. DOI:10.1093/pcp/pcy071.
You may like
Related articles And Qustion
Lastest Price from 9-Fluorenylmethyl chloroformate manufacturers

US $750.00/kg2025-08-04
- CAS:
- 28920-43-6
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 50tons

US $45.00/kg2025-04-21
- CAS:
- 28920-43-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons