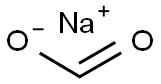The Versatile Compound: Sodium Formate in Modern Chemistry
Sodium formate usually appears as a white deliquescent powder, it is utilized in the production of sodium hydrosulphite, an important industrial reducing agent.

Synthesis of Sodium Formate
For commercial use, sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 130 °C and 6-8 bar pressure:
CO + NaOH → HCO2Na
Because of the low-cost and large-scale availability of formic acid by carbonylation of methanol and hydrolysis of the resulting methyl formate, sodium formate is usually prepared by neutralizing formic acid with sodium hydroxide.[1]
Sodium formate is also unavoidably formed as a by-product in the final step of the pentaerythritol synthesis and in the crossed Cannizzaro reaction of formaldehyde with the aldol reaction product trimethylol acetaldehyde [3-hydroxy-2,2-bis(hydroxymethyl)propanal].
Applications of Sodium Formate
Sodium Formate is often used as a deicer for runways and roads in temperate and continental climate zones. Furthermore, it can be used as a dyeing agent for printing or a bleaching agent for cotton lint in textile and fabric applications; In India and Brazil where the leather industry plays an important role, sodium formate is often used as a tanning agent for leather tanning.
Sodium formate is basic in aqueous solution of weak acid (formic acid) and strong base (sodium hydroxide). In chemical reactions, it can be used as a buffering agent to increase pH levels, a reducing agent for precious metals, a reagent for the determination of phosphorus and arsenic and as a mordant.
References
[1] Arnold Willmes, Taschenbuch Chemische Substanzen, Harri Deutsch, Frankfurt (M.), 2007.
See also
Lastest Price from Sodium formate manufacturers

US $1200.00-1100.00/ton2025-08-12
- CAS:
- 141-53-7
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
US $2.00-5.00/kg2025-06-18
- CAS:
- 141-53-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg