The Versatile Applications and Production of Propylene Carbonate
Introduction
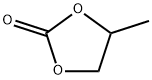
Propylene carbonate is a dipolar aprotic solvent that has previously found application mainly in extractions, electrochemistry, cosmetics, and medicine. In addition to its excellent solvation properties, PC has valuable physical properties such as low viscosity, and it is essentially odorless. Like other organic carbonates PC is usually anhydrous, noncorrosive, nontoxic, and biodegradable. Based on these properties, organic carbonates offer a "safe" and environmentally friendly alternative to standard solvents such as CH2Cl2 and THF, as well as aromatic and toxic solvents. Many alkyl carbonates are available commercially. This article will introduce its polymerization method and uses as solvent.1

Figure 1 Characteristics of Propylene carbonate
Polymerization
Polymerization of propylene carbonate was carried out at 120-180℃ mainly with the use of diethylzinc catalyst. The polymer was a pale-yellow, viscous material of relatively low molecular weight (1000-4000).
Polymerization of propylene carbonate was usually carried out in a 50-ml stainless steel autoclave. Measured quantities of propylene carbonate, solvent, and catalyst were placed in the autoclave under nitrogen. The autoclave was cooled by liquid nitrogen and was degassed in uacuo; it was then heated to the reaction temperature and kept standing in an oil bath equipped with temperature regulator. Carbon dioxide was introduced through a wet gas meter and drying towers containing calcium chloride and phosphorus pentoxide. After the reaction, the reaction mixture was washed with a dilute hydrochloric acid-methanol solution, and the polymer produced was precipitated by adding large volumes of water. The precipitate was then dried in uacuo overnight at room temperature and weighed.2
Application
Propylene carbonate (4-methyl-1,3-dioxolane-2-one)is an excellent solvent for the extraction of ferroin-type iron(II) complexes from aqueous solution. The solvent has the characteristics normally desired in an extractant: it is colourless, denser than water, non-toxic, almost odourless, and has little tendency to form emulsions. Propylene carbonate is partially soluble in water; however, aqueous solutions can be rapidly and predictably saturated with the solvent. At 24 °C the solubility of propylene carbonate in water is 0.254 g ml-1 and in 2.7 M sodium chloride 0.125 g ml-1.
It is noteworthy that with the increased concern over the nature and quality of man's environment, little solicitude has been expressed regarding the routine use of highly toxic solvents, such as benzene, diethyl ether, chloroform, carbon tetrachloride and nitrobenzene, as extractants or solvents in analytical chemistry. Propylene carbonate is non-toxic and consequently is preferred as an extractant to these toxic solvents.3
References:
[1] JEROME BAYARDON DR. Propylene Carbonate as a Solvent for Asymmetric Hydrogenations?[J]. Angewandte Chemie International Edition, 2007, 46 31: 5807-5983. DOI:10.1002/anie.200700990.[2] KAZUO SOGA. Polymerization of propylene carbonate[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1977, 15 1: 1-254. DOI:10.1002/pol.1977.170150120.
[3] B G STEPHENS. Propylene carbonate extraction of tris(pentan-2,4-dione)-iron(3) from aqueous solution: application to the spectrophotometric determination of iron.[J]. Analyst, 1971, 96 140. DOI:10.1039/an9719600230.
You may like
Related articles And Qustion
Lastest Price from Propylene carbonate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 108-32-7
- Min. Order:
- 250KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 108-32-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 TON