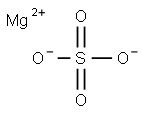The Versatile Compound: Exploring the Synthesis, Components, Applications, and Storage of Magnesium Sulfate
Introduction
Magnesium sulfate, commonly referred to as Epsom salt, is a chemical compound with the formula MgSO₄. This inorganic salt contains magnesium, sulfur, and oxygen and is highly soluble in water. In the realm of chemistry and various industries, magnesium sulfate plays a pivotal role due to its multifaceted applications, ranging from agricultural to medical uses[1].

Figure 1 Characteristics of Magnesium sulfate
Synthesis Method
The synthesis of magnesium sulfate typically involves several methods, depending on the desired purity and application. The most common industrial method includes the reaction of magnesium carbonate (MgCO₃) or magnesium hydroxide (Mg(OH)₂) with sulfuric acid (H₂SO₄). Alternatively, magnesium sulfate can be produced by the reaction of magnesium oxide (MgO) with sulfuric acid For laboratory-scale synthesis, magnesium sulfate heptahydrate (MgSO₄·7H₂O) can be prepared by dissolving magnesium hydroxide in sulfuric acid and allowing the solution to crystallize. The crystalline form, known as Epsom salt, is widely recognized for its purity and ease of handling.
Main Components
Magnesium sulfate consists of magnesium cations (Mg²⁺) and sulfate anions (SO₄²⁻). The most common form encountered is magnesium sulfate heptahydrate (MgSO₄·7H₂O), which includes seven molecules of water of crystallization. This crystalline form is highly soluble in water, making it versatile for a wide range of applications, including medical treatments, agricultural fertilizers, and chemical processes. The compound’s solubility and hydration state are critical to its effectiveness in various uses. Additionally, the anhydrous form of magnesium sulfate (MgSO₄) is utilized in specific industrial processes, particularly where the presence of water needs to be minimized to maintain the integrity of reactions or products. This form is essential in applications requiring a desiccant or in environments where moisture control is critical.
Applications
Agriculture: Magnesium sulfate is widely used as a fertilizer to correct magnesium deficiencies in soil, essential for chlorophyll production in plants. It promotes nutrient absorption and enhances crop yield. It is particularly beneficial for crops like tomatoes, peppers, and roses.
Medical: In the medical field, magnesium sulfate serves several crucial functions. It is used as an electrolyte replenisher and is administered intravenously to treat eclampsia in pregnant women, severe asthma attacks, and cardiac arrhythmias. Epsom salt baths are a popular home remedy for muscle soreness and inflammation due to the compound’s anti-inflammatory properties.
Pharmaceuticals: In the pharmaceutical industry, magnesium sulfate is incorporated into formulations of laxatives and antacids. It acts as an osmotic laxative, drawing water into the intestines to relieve constipation. Its role in antacids helps neutralize stomach acid, providing relief from indigestion and heartburn.
Storage Methods
Proper storage of magnesium sulfate is crucial to maintain its efficacy and prevent degradation. The compound should be stored in a cool, dry place, away from direct sunlight and moisture, as exposure to these elements can compromise its chemical stability. Containers should be tightly sealed to avoid exposure to humidity, which can lead to clumping or dissolution, particularly in the case of the anhydrous form. For large-scale storage, magnesium sulfate should be kept in airtight, moisture-resistant containers, preferably in well-ventilated areas to prevent the buildup of any harmful fumes. It is also advisable to label storage containers clearly to avoid any accidental misuse or contamination. In laboratory settings, magnesium sulfate heptahydrate should be stored in glass or plastic containers with tight lids to minimize moisture absorption from the air, ensuring its purity and effectiveness for experimental use. Care should be taken to avoid contamination with other chemicals, which could alter its properties or reactivity, ensuring it remains fit for its intended applications. Regular inspections of storage conditions and containers can further help in maintaining the quality of magnesium sulfate over time.
Conclusion
Magnesium sulfate stands as a cornerstone in various fields, thanks to its diverse applications and chemical properties. From agriculture to medicine and industrial processes, its importance cannot be overstated. Understanding its synthesis, main components, applications, and proper storage methods is crucial for professionals working with this versatile compound. Whether used as a fertilizer, medical treatment, industrial agent, or chemical synthesis, magnesium sulfate continues to be an invaluable resource in the advancement of numerous scientific and practical domains[2].
![Article illustration]() References
References
[1]Euser, Anna G., and Marilyn J. Cipolla. "Magnesium sulfate for the treatment of eclampsia: a brief review."Stroke40.4 (2009): 1169-1175.
[2]Širvinskas, Edmundas, and Rokas Laurinaitis. "Use of magnesium sulfate in anesthesiology."Medicina (Kaunas)38.7 (2002): 695-698.
Related articles And Qustion
Lastest Price from Magnesium sulfate manufacturers

US $1200.00-1100.00/ton2025-09-23
- CAS:
- 7487-88-9
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $10.00/KG2025-04-21
- CAS:
- 7487-88-9
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt