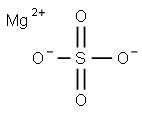Application and Pharmacokinetics of Magnesium sulfate
General description
Magnesium sulfate is a magnesium salt having sulfate as the counterion. It has a role as an anticonvulsant, a cardiovascular drug, a calcium channel blocker, an anaesthetic, a tocolytic agent, an anti-arrhythmia drug, an analgesic and a fertilizer. It is a magnesium salt and a metal sulfate[1].
Figure 1 The chemical structure of magnesium sulfate
Application and Pharmacokinetics
1. It can be used as an anticonvulsant and is ommonly used in pregnancy-induced hypertension. It can also be used to lower blood pressure, treat pre-eclampsia and eclampsia and preterm labor. Industrially used in tanning, explosives, fertilizers, papermaking, porcelain, printing dyes, lead-acid batteries and other industries.In agriculture and horticulture, magnesium sulfate is used to improve magnesium deficiency in soils . This is most commonly used for magnesium sulfate potted plants, or magnesium-starved crops such as potatoes, roses, tomatoes, peppers, and hemp. Feed grade magnesium sulfate as a magnesium supplement in feed processing[1].
2. Onset of action is 20 minutes after intramuscular injection and almost immediate effect when administered intravenously. The effect lasts for 30 minutes, and the effective blood magnesium concentration is 2-3.5mmol/L in the treatment of pre-eclampsia and eclampsia, and the effective blood magnesium concentration in the treatment of preterm birth is 2.1-2.9mmol/L, with large individual differences. Intramuscular and intravenous injection, the drug is excreted by the kidneys, and the rate of excretion is related to blood magnesium concentration and glomerular filtration rate.
Mechanism of Action
1. Magnesium sulfate is easily soluble in water, but not absorbed when taken orally. Magnesium ions and sulfate ions in the aqueous solution are not easily absorbed by the intestinal wall, which increases the osmotic pressure in the intestine and moves the water in the body fluid to the intestinal lumen, increasing the volume of the intestinal lumen. Dilation of the intestinal wall, thereby stimulating the afferent nerve endings of the intestinal wall, reflexively causing increased peristalsis and catharsis
2. Magnesium sulfate can stimulate the duodenal mucosa, reflexively cause the relaxation of the sphincter of the common bile duct and the contraction of the gallbladder, thereby promoting the emptying of the gallbladder and promoting the effect of gallbladder.
3. Magnesium ions can inhibit the activity of the central nervous system, inhibit the release of acetylcholine at the motor nerve-muscle junction, block the conduction at the neuromuscular junction, reduce or relieve muscle contraction, and at the same time have a relaxing effect on vascular smooth muscle, so that the spasm peripheral blood vessels dilate, lower blood pressure, thus have preventive and therapeutic effects on eclampsia.Magnesium ions act directly on uterine muscle cells, antagonize the effect of calcium ions on uterine muscle contraction, and inhibit uterine contraction
Synthesis
It is obtained by decomposing or neutralizing magnesium oxide, magnesium hydroxide, magnesium carbonate, magnesite, etc. as raw materials with sulfuric acid. There are also by-products of potassium chloride production as raw materials, mixed with magnesium-containing mother liquor after bromine production in proportion, cooled and crystallized to obtain crude magnesium sulfate, and then heated and filtered, impurity removal, and cooling and crystallized to obtain industrial magnesium sulfate. It can also be obtained by heating and concentrating bittern, crystallization and separation, or by carbonization of magnesium oxide and gypsum aqueous suspension.
Storage and Safety
1. It should be stored in a cool, ventilated warehouse. Keep it away from fire and heat sources. Protect from direct sunlight. It needs to be packaged tightly and should be kept away from oxidizer, do not store together. Storage areas should be provided with suitable materials to contain spills.
2. The dust of this product has a stimulating effect on mucous membranes, and long-term exposure can cause respiratory tract inflammation. Mistaken ingestion has cathartic effect, if there is renal dysfunction, it can cause magnesium poisoning, causing stomach pain, vomiting, watery diarrhea, collapse, dyspnea, cyanosis, etc.It is harmful to the environment and can cause pollution to water bodies.This product is non-flammable and irritating[2].
References
1.Scientists successfully analyze the new phase structure of magnesium oxysulfide cement. Chinese Academy of Sciences.
2.Durlach J, Guiet-Bara A, Pagès N, Bac P, Bara M. Magnesium chloride or magnesium sulfate: a genuine question. Magnes Res. 2005 Sep;18(3):187-92.
Related articles And Qustion
See also
Lastest Price from Magnesium sulfate manufacturers

US $1200.00-1100.00/ton2025-09-23
- CAS:
- 7487-88-9
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $10.00/KG2025-04-21
- CAS:
- 7487-88-9
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt