The uses of Guaiacol
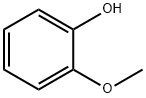
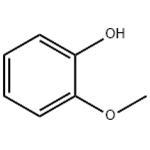
Guaiacol is a naturally-occurring organic compound with the formula C6H4(OH)(OCH3), first isolated by Otto Unverdorben in 1826. Although it is biosynthesized by a variety of organisms,this yellowish aromatic oil is usually derived from guaiacum or wood creosote. Samples darken upon exposure to air and light. Guaiacol is present in wood smoke, resulting from the pyrolysis of lignin. The compound contributes to the flavor of many substances such as whisky and roasted coffee.
Uses
Guaiacol is a precursor to various flavorants, such as eugenol and vanillin.An estimated 85% of the world's supply of vanillin comes from guaiacol. The route entails condensation of glyoxylic acid with guaiacol to give mandelic acid, which is oxidized to produce a phenylglyoxylic acid. This acid undergoes a decarboxylation to afford vanillin.
Guiacol, i.e. o-hydroxyanisole, gives a distinct color reaction with U(VI) suitable for spectrophotometric determination of the metal. The complex formed in the reaction has an absorption maximum at 352 nm. Optimum pH for the color development ranges from 6.5 to 8.5. The molar absorptivity and Sandell's sensitivity of the method were found to be 3.75×103 l·mol−1·cm−1 and 0.063 μg·cm−2, respectively. Many anions and cations do not interfere up to 100 ppm. The method has been made very specific by selective extraction of U(VI) with TBP from a mixture of different cations and anions in the presence of 60% NH4NO3 as salting out agent followed by developing the color in the non-aqueous phase by adding quaiacol in methanol at pH 6.5 to 8.5 An amount as low as 30 μg of uranium (VI) per 10 ml of the solution could be satisfactorily determined with an RSD of ±2.0%. The method was applied to rock samples after U(VI) had been extracted from a sample solution into 25% TBP in hexane. Results obtained by the new method compare very well with those of conventional fluorimetric and radiometric assays. The features of the method include excellent precision, rapidity, good selectivity, and ease of performance.
The unprocessed bio-oil obtained by the pyrolysis of lignocellulosic biomass comprises hundreds of oxy-components which vitiate its quality in terms of low heating value, low stability, low pH, etc. Therefore, it has to be upgraded prior to its use as transportation fuel. In this work, guaiacol, a promising compound of the phenolic fraction of unprocessed bio-oil, is considered as a model component for studying its hydrodeoxygenation over a Pt3 catalyst cluster. The production of catechol, 3-methylcatechol, m-cresol and o-cresol from guaiacol over a Pt3 cluster is numerically investigated using density functional theory. Further, the kinetic parameters are obtained over a wide range of temperature, i.e. 473–673 K at an interval of 50 K. Briefly, results indicate that O─H and C─H bond scissions determine the reaction rates of ‘guaiacol to catechol’ and ‘catechol to 3-methylcatechol’ reactions with activation energies of 30.32 and 41.3 kcal mol−1, respectively. On the other hand, C─O bond scissions determine the rates of 3-methylcatechol to m- and o-cresol production reactions, respectively.
You may like
Related articles And Qustion
Lastest Price from Guaiacol manufacturers
US $0.00/kg2025-09-10
- CAS:
- 90-05-1
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $0.00/kg2025-05-15
- CAS:
- 90-05-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS