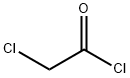
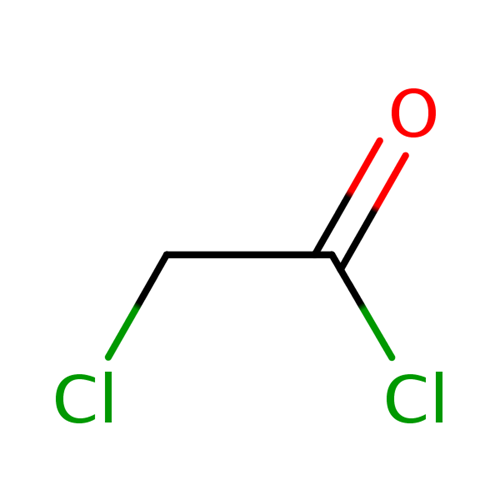
The uses of chloroacetyl chloride
Chloroacetyl chloride, a colorless to yellow liquid, is a bi-functional compound that is useful as a chemical building block. This chemical is mainly used in the production of herbicides, in the formulation of active pharmaceutical ingredients, and in the production of other useful chemicals.
Uses
The main use of chloroacetyl chloride is in the manufacturing of herbicides used in agriculture. Especially, popular herbicides such as alachlor and butachlor are manufactured using chloroacetyl chloride. However, due to the fact that such herbicides create environmental pollution, production and use of alachlor and butachlor has been banned in the EU region.
However, several countries in Latin America and in the Asia Pacific region continue the use of such herbicides and therefore the demand of these herbicides is steady and rising. This is expected to boost the growth of the global chloroacetyl chloride market. In case of medical emergencies, the human body is unable to produce the adrenalin hormone. Thus, demand for externally produced adrenalin is expected to increase due to an increasing risk of life threatening diseases. Chloroacetyl chloride is used in the production of epinephrine (adrenalin hormone). This is also expected to fuel the growth of the global chloroacetyl chloride market over the forecast period.
Chloroacetyl chloride occasionally serves as a chlorinating agent. 2,4-Pentanedione was chlorinated at the 1- and 5-positions upon treatment with ClCH2COCl/AlCl3 in the presence of Cu(OAc)2. a,b Chloroacetyl chloride has also been used to transform 2-picoline N-oxide and 2-oxo-2-dimethylamino-5-methyl-1,2-oxophospholane to 2-chloromethylpyridine and 2-oxo-2-chloro-5-methyl-1,2- oxophospholane, respectively.
Reactions
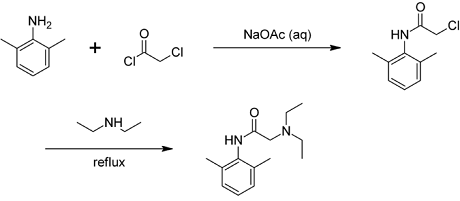
Chloroacetyl chloride is bifunctional—the acyl chloride easily forms esters and amides, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine is illustrative:
Safety
Like other acyl chlorides, reaction with other protic compounds such as amines, alcohols, and water generates hydrochloric acid, making it a lachrymator.
There is no regulated permissible exposure limit set by the Occupational Safety and Health Administration. However, the National Institute for Occupational Safety and Health has set a recommended exposure limit at 0.05 ppm over an eight-hour work day.
Related articles And Qustion
See also
Lastest Price from Chloroacetyl chloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 79-04-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/kg/drum2025-04-21
- CAS:
- 79-04-9
- Min. Order:
- 250kg/drum
- Purity:
- 99%
- Supply Ability:
- 100000MT