o-Xylene: Occurence, Uses, Environmental Fate, Toxicity
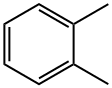
o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2. with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). o-Xylene is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colorless slightly oily flammable liquid[1]. O-xylene appears as a colorless watery liquid with a sweet odor. Less dense than water. Insoluble in water. Irritating vapor. o-Xylene belongs to the family of Toluenes, which are compounds containing a benzene ring which bears a methane group.
Petroleum contains about one weight percent xylenes. Most o-xylene is produced by cracking petroleum, which affords a distribution of aromatic compounds, including xylene isomers. m-Xylene is isomerized to o-xylene. Net production was approximately 500,000 tons in the year 2000. o-Xylene is largely used in the production of phthalic anhydride, which is a precursor to many materials, drugs, and other chemicals. Xylenes are not acutely toxic, for example the LD50 (rat, oral) is 4300 mg/kg. Effects vary with animal and xylene isomer. Concerns with xylenes focus on narcotic effects.
In pregnant rats, o-xylene has been shown to cross the placenta. The concentrations in fetal blood were 25-30% of that in maternal blood after a 2-hr exposure, o-Xylene was also detected in amniotic fluid.
o-Xylene is an important commercial chemical used to make other chemicals, pharmaceuticals, dyes, insecticides and in gasoline blending. o-Xylene is a component in many household paints and automotive exterior and engine maintenance products. It is typically found in a mixture with other xylenes (3- and 4-xylene).
Workers that use o-Xylene may breathe in vapors or have direct skin contact. The general population may be exposed by breathing in air, contact with consumer products containing xylenes (gasoline, paints, varnishes, paint thinner, etc.), smoking cigarettes, and consumption of food and drinking water. If o-Xylene is released to the environment, it will be broken down in air. o-Xylene is not expected to be broken down by sunlight. o-Xylene will move into air from moist soil and water surfaces. o-Xylene is expected to move moderately through soil. o-Xylene will be broken down by microorganisms, and is not expected to build up in fish. RISK: Risks discussed below are for xylene mixtures in general, as o-Xylene is most often found in a mixture with 3- and 4-xylene. Studies indicate that risk of toxicity is the same for 2-, 3-, and 4-xylene, or a mixture of the three chemicals. Xylenes are skin, eye, nose, and throat irritants. Nervous system effects (headache, dizziness, confusion, incoordination, impaired balance, forgetfulness, etc.) are the primary effects observed in humans that breathe high levels of xylenes. Difficulty breathing, nausea, and damage to the lungs, liver, and kidneys have also been observed following exposure to high vapor levels.
Unconsciousness and even death may occur at very high levels. Similar effects were noted in laboratory animals exposed to moderate-to-high levels of xylenes. Studies on the potential for xylenes to cause infertility, abortion, or birth defects in humans are considered inadequate to assess risk due to simultaneous exposure to other solvents (e.g. benzene). Abortion and delayed growth and development of offspring were observed in laboratory animals following exposure to xylene during pregnancy, but only at doses that were toxic to the mothers. Infertility and major birth defects were not observed in laboratory animals following exposure before and/or during pregnancy. No specific forms of cancer have been specifically associated with xylene exposure in workers exposed to solvent mixtures (including xylenes). No evidence of cancer was observed in laboratory animals following lifetime oral exposure to xylenes. The U.S. EPA IRIS program determined that data are inadequate for an assessment of the human carcinogenic potential of xylenes. The International Agency for Research on Cancer has determined that xylenes are not classifiable as to their carcinogenicity to humans based on lack of adequate human data and inconclusive animal data. The potential for xylene to cause cancer in humans has not been assessed by the U.S. National Toxicology Program 13th Report on Carcinogens.
References
1. J. P. Leineweber, Solubility of fibres in vitro and in vivo. In T. Guiter, ed., Biological Effects of Man-Made Mineral Fibers
(Proc. WHO/IARC Conf.), Vol. 2,World Health Organization, Copenhagen, 1984, pp. 87–101.
2. Bellmann et al., Persistence of man-made mineral fibres (MMMF) and asbestos in rat lungs. Ann. Occup. Hyg. 30, 693–709 (1987).
3. Eastes et al., Estimating rock and slag wool fiber dissolution rate from composition. Inhal. Toxicol. 12, 127–1139 (2000).
Related articles And Qustion
Lastest Price from o-Xylene manufacturers

US $19.90/kg2025-04-21
- CAS:
- 95-47-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.00/KG2024-10-11
- CAS:
- 95-47-6
- Min. Order:
- 1KG
- Purity:
- 99.9
- Supply Ability:
- 1 ton