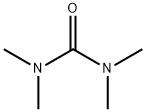
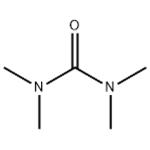
The uses and toxicity of 1,1,3,3-Tetramethylurea
Description
Tetramethylurea (TMU; 1,1,3,3-tetramethylurea) is a colorless liquid used as a solvent in industrial applications. It is a much stronger donor solvent than water and, thus, would be expected to preferentially solvate with cations over water. TMU is frequently used as an acid scavengers, and water was generally removed by Drierite® and molecular sieves during glycosidation reactions.
Uses
The dielectric properties of water can be effectively modulated by adding solutes, such as ions, sugar, and organic compounds. 1,1,3,3-Tetramethylurea (TMU) is an amphiphilic molecule compound. This compound contains both hydrophobic methyl groups and hydrophilic ether groups. The presence of TMU can modify the dielectric relaxation process of water because various hydration shells can form around the methyl and ether groups. Compared with pure water, the real part of the permittivity of a TMU solution is lower, and the imaginary part is higher within a low-frequency region, making a TMU/water mixture a good option for an absorber inclusion to obtain a PA band within the low microwave band. Zhang et al. expand the absorption region of a water-based absorber by introducing TMU into the inclusion, whose dielectric properties are tunable through the concentration of TMU[1].
Since Zn-metal anode suffers from continuous parasitic reactions, random dendrite growth, and sluggish kinetics in aqueous electrolytes, Yang et al. introduced a high donor number solvent (TMU) as an electrolyte additive to enable highly reversible Zn-metal anode, where the TMU can:
Preferentially adsorb on the Zn surface to inhibit Zn corrosion and suppress parasitic reaction.
Solvate with Zn2+ and exclude the H2O from the Zn2+ solvation sheath to weaken water activity significantly.
Contribute to forming an inorganic-organic bilayer solid electrolyte interphase to enable homogeneous and rapid Zn2+ transport kinetics for dendrite-free Zn deposition[2].
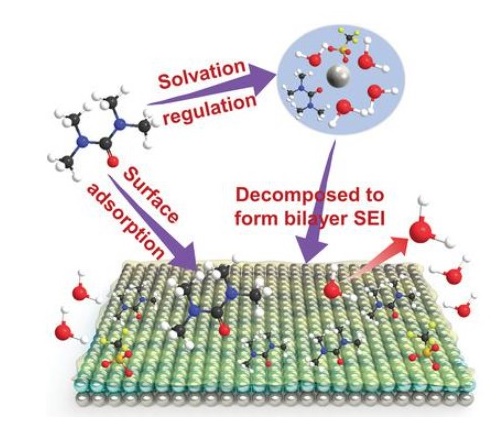
Benefiting these three merits, the resulting aqueous electrolyte demonstrates a highly reversible Zn plating/stripping cycling in a Zn||Ti asymmetric cell for over 1200 cycles and in a Zn||Zn symmetric cell for over 4000 h.
Toxicity
TMU has been shown to have slight acute oral toxicity. In male rats, the lowest lethality (ALD) dose was 2250 mg/kg. LD50 values of 800 mg/kg and 2920 mg/kg were determined for male rats and mice, respectively[3]. Slight to moderate toxicity was observed following acute dermal exposures to TMU. The dermal LD50 in albino rabbits was 3160 mg/kg. The 24-hour dermal ALD in male albino rabbits was determined to be 5000 mg/kg. In guinea pigs, dermal or intradermal exposure caused irritation; skin sensitization did not occur.
Regarding acute ocular toxicity, serious corneal, iritic, and conjunctival injury was produced in rabbits. Rats exposed by inhalation to 1480 mg/m3 (312 ppm) for 4 hours died within 2 days of exposure. Clinically, the rats were unresponsive to external stimuli and showed irregular respiration.
References
[1] Jiaqi Zhang. “Ultra-broadband microwave metamaterial absorber with tetramethylurea inclusion.” Optics express 27 18 1 (2019): 25595–25602.
[2] Jianghong Yang. “Three Birds with One Stone: Tetramethylurea as Electrolyte Additive for Highly Reversible Zn-Metal Anode.” Advanced Functional Materials 32 49 (2022).
[3] S. Munley. “DEVELOPMENTAL TOXICITY STUDY OF TETRAMETHYLUREA (TMU) IN RATS.” Drug and Chemical Toxicology 1 1 (2001): 259–271.
See also
Lastest Price from 1,1,3,3-Tetramethylurea manufacturers
US $1.50/g2025-09-12
- CAS:
- 632-22-4
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 100 Tons

US $0.00-0.00/KG2025-04-21
- CAS:
- 632-22-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt