Biodegradation of cellulose acetate
Background
The accumulation of vast piles of plastic waste in landfills and natural environments, especially in the sea and coastal regions, is one of the most significant environmental concerns facing us. Millions of tons of plastic waste are floating in the oceans worldwide every year due to improper disposal, unintentional discarding of things like fishing gear, and spillage into oceans caused by wind and rain, which has adverse effects on ecosystems. The prolonged degradation of plastic waste under natural environmental conditions calls for immediate action. However, no single solution can solve the whole plastic waste problem.
Chemical properties and applications
Mentioning the above issues, we should consider a biodegradable material, cellulose acetate. This compound is more biodegradable than petroleum resins and can biodegrade even in seawater. Cellulose acetate (CA) is a widely used chemically modified natural polymer considered a semi-synthetic polymer. Its applications vary from the textile industry to plastic films, packaging, and cigarette filter tows. It is an eco-friendly material and majorly sourced from cellulose in wood or cotton linters through reaction with acetic acid. Cellulose is a natural biopolymer and inherently degradable in suitable natural environments. CA is produced from cellulose through acetylation of some of the hydroxyl groups. The favorable properties of CA include hardness, good impact resistance, optical transparency, resistance to hydrocarbons, and lack of static electricity[1].
Biodegradation
Notably, there has been some debate about the definition of biodegradation. One view is that biodegradation is the microbial-initiated conversion of a substrate in a biologically active environment into carbon dioxide (aerobically), methane (anaerobically), cell wall material, and other biological products. Another view requires a specific degradation rate, such as weight loss versus time.
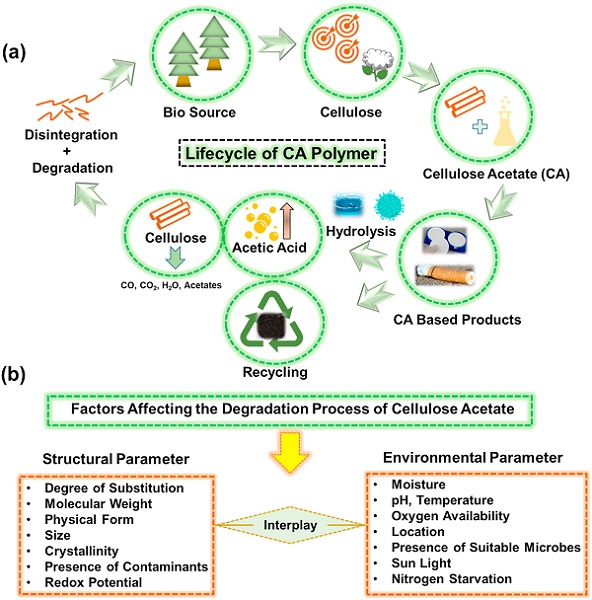
CA is generally recognized as a biodegradable polymer within the scientific community. The biodegradation rate is governed by the degree of acetyl-substitution (DS) and the actual conditions the material is subjected to. The biodegradation rate decreases as the function of increasing DS. In addition to DS, molar mass, crystallinity, physical form, the presence of contaminants, and electron acceptors have been reported to control the degradation rate. Numerous “environmental factors” combined with the structural features further influence the degradation rate at any given location, including temperature, humidity, pH, sunlight, and availability of oxygen, nutrients, and microorganisms.
Cellulose is readily biodegraded by organisms that utilize cellulase enzymes, but due to the additional acetyl groups, cellulose acetate requires the presence of esterases for the first step in biodegradation. Once partial deacetylation has been accomplished either by enzymes or by partial chemical hydrolysis, the polymer’s cellulose backbone is readily biodegraded. Cellulose acetate is photo-chemically degraded by UV wavelengths shorter than 280 nm but has limited photo degradability in sunlight due to the lack of chromophores for absorbing ultraviolet light[2].
References
[1] Nisha Yadav, Minna Hakkarainen. “Degradable or not? Cellulose acetate as a model for complicated interplay between structure, environment and degradation.” Chemosphere 265 (2021): Article 128731.
[2] Juergen Puls, Dirk Hölter, Steven A. Wilson. “Degradation of Cellulose Acetate-Based Materials: A Review.” Journal of Polymers and the Environment 19 1 (2010): 152–165.
You may like
Related articles And Qustion
See also
Lastest Price from Cellulose acetate manufacturers

US $1000.00-400.00/g2025-04-21
- CAS:
- 9004-35-7
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 5000

US $180.00/kg2025-04-15
- CAS:
- 9004-35-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton


