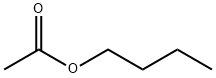
The toxicity of n-Butyl acetate
Introduction
N-butyl acetate, n-butanol, n-butyraldehyde, and n-butyric acid (the butyl series) are a family of metabolically related compounds linked by an industrial production method relying on sequential oxidation/reduction reactions[1].
N-Butyl acetate (nBA) is a clear, volatile organic solvent with consumer and non-consumer uses. It is used in photographic film, nail polish removers, lacquers, perfumes, oils, and resins. The 1993 estimated production value in the US was 130,000 metric tons, with more than 90% used as a solvent in the coatings industry. This solvent has been used for many years, with few reports of harmful effects on humans.

Chemical property
n-Butyl Acetate is a clear, colorless liquid with a fruity odor. It is miscible with all conventional solvents such as alcohols, ketones, aldehydes, ethers, glycols, glycol ethers, aromatic hydrocarbons, and aliphatic hydrocarbons. Its solubility in water is limited.
Acute toxicity
In a 1986 study, n-butyl acetate aerosol was found to be of high acute toxicity to rats (4-hour LCs0 160 ppm). In a series of follow-up studies at three laboratories (including the one that generated the initial value), only two managed to generate LC50 values 390 ppm and 1096 ppm, moderate and low toxicity, respectively), while four others reported no deaths at concentrations of up to 1034 ppm, 4429 ppm (two studies) or 9312 ppm. A summary of these investigations has now been published. It has been estimated that no more than 20% of total n-butyl acetate exposure in the workplace is aerosol. The investigators expressed the view that the low and variable LC50 values for the aerosol were not of great toxicological concern in occupational settings[2].
Subchronic toxicity
The subchronic toxicity of n-butyl acetate (nBA), a common industrial solvent, was tested in rats in a 13-week inhalation study. Male and female Sprague–Dawley (SD) rats were exposed to concentrations of 0, 500, 1500, or 3000 ppm nBA for 6 h per day, 5 days per week for 13 consecutive weeks. Transient signs of sedation were observed only during exposure to the 1500 and 3000 ppm concentrations. Body weights for the 1500 and 3000 ppm groups were significantly reduced. Feed consumption values for the 1500 and 3000 ppm groups were significantly lower than the control group. Weights of the liver, kidneys, and spleen were significantly lower for the 3000 ppm male group; testes and adrenal gland weights for the 1500 and 3000 ppm groups and the lung weight for the 3000 ppm male group were significantly higher than for the control group. Signs of glandular stomach irritation and necrosis in the non-glandular stomach were observed in 3000 ppm female rats. Degeneration of the olfactory epithelium along the dorsal medial meatus and ethmoturbinates of the nasal passages of some 1500 and all 3000 ppm rats was also seen. The severity was mild to moderate for the 3000 ppm group and minimal to mild for the 1500 ppm group. No effects were observed in the lungs of any group. The no-observed-effect level (NOEL) for this study is considered to be 500 ppm. The data presented here are relevant to the toxicity risk assessment of n-butanol due to the rapid hydrolysis of nBA in vivo[3].
Health hazard
n-Butyl Acetate can affect you by ingestion and may be absorbed through the skin. Contact can severely irritate and burn the skin and eyes. Prolonged or repeated contact can cause a skin rash, dryness, and redness. Inhaling n-Butyl Acetate can irritate the nose, throat andlungs. Exposure can cause headaches, dizziness, lightheadedness, and passing out. n-Butyl Acetate may affect the nervous system. n-Butyl Acetate is a flammable liquid and a Dangerous fire hazard.
References
[1] J G Teeguarden. “Derivation of a human equivalent concentration for n-butanol using a physiologically based pharmacokinetic model for n-butyl acetate and metabolites n-butanol and n-butyric acid.” Toxicological Sciences 85 1 (2005): 429–46.
[2] “What is the acute inhalation toxicity of n-butyl acetate in rats?” Food and Chemical Toxicology 35 10 (1997): Page 1133.
[3] R.M David . “Evaluation of subchronic toxicity of n-butyl acetate vapor.” Food and Chemical Toxicology 39 8 (2001): Pages 877-886.
You may like
Related articles And Qustion
See also
Lastest Price from Butyl acetate manufacturers
US $10.00/KG2025-04-21
- CAS:
- 123-86-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 123-86-4
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons