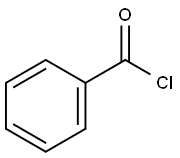
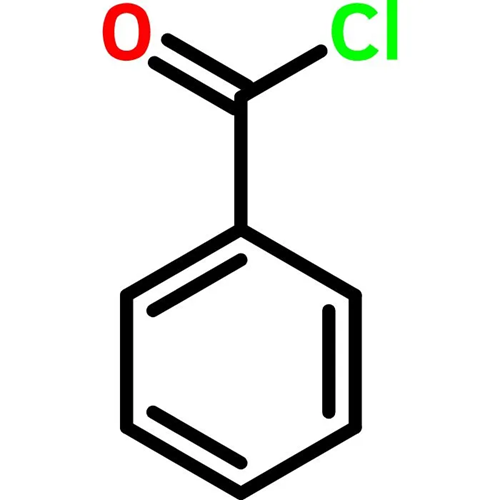
How is Benzoyl chloride converted to benzaldehyde?
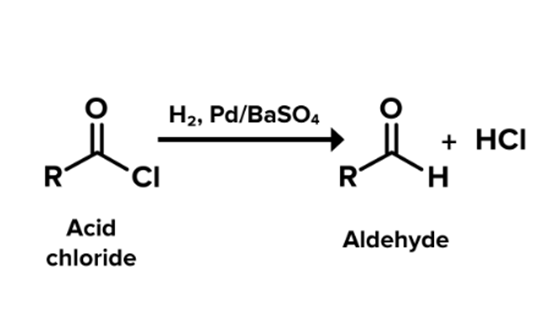
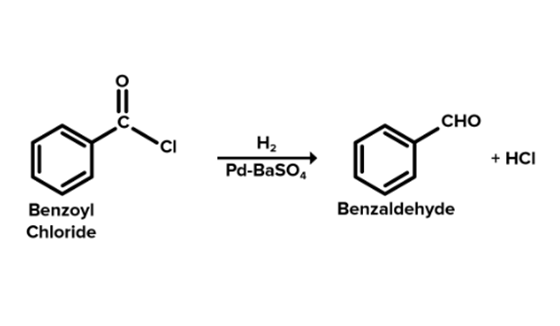
Benzoyl chloride is converted into benzaldehyde by the Rosenmund reaction. The Rosenmund reaction is a hydrogenation process in which hydrogen molecules react with chloride in the presence of a catalyst (palladium on barium sulphate) to form an aldehyde. The reaction is shown in Figure 1 below. The Rosenmund catalyst (palladium on barium sulfate) is prepared by the reduction of palladium(II) chloride solution with reducing agent formaldehyde in the presence of barium sulfate. When benzoyl chloride is hydrogenated in the presence of palladium on barium sulphate (Pd/BaSO4), a reduction reaction occurs to produce benzaldehyde and hydrochloric acid. The reaction process is shown in Figure 2. Therefore, Benzaldehyde and by product hydrochloric acid is formed from Benzoyl chloride by the Rosenmund reaction.
You may like
Related articles And Qustion
See also
Lastest Price from Benzoyl chloride manufacturers

US $6.00-1.80/KG2025-08-07
- CAS:
- 98-88-4
- Min. Order:
- 1000KG
- Purity:
- 99.5 %
- Supply Ability:
- 100 mt
US $0.00/kg2025-05-21
- CAS:
- 98-88-4
- Min. Order:
- 1000kg
- Purity:
- 99
- Supply Ability:
- 20000MT