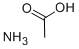ChemicalBook > Articles Catagory List >API >is-ammonium-acetate-an-acid-or-base-and-what-is-the-value-of-ph
Is Ammonium acetate an acid or base and what is the value of pH?
Apr 28,2024
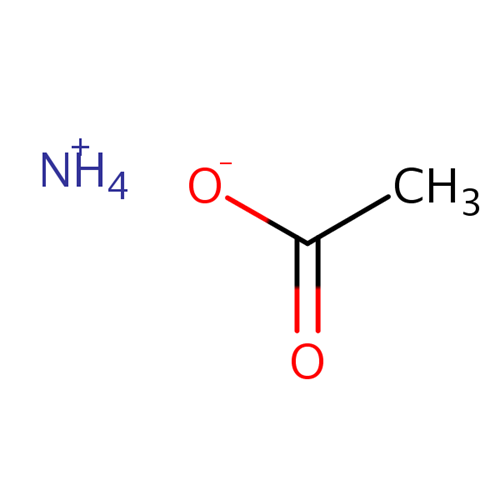
Ammonium acetate is a salt that is a combination of a weak acid and a weak base. When dissolved in water, ammonium acetate has a pH of 7. Ammonium acetate hydrolyses to form ammonia and acetic acid, which dissociate in roughly equal amounts, but this pH is very unstable. Ammonium acetate has a buffering effect at pH values around 4.75 (pKa for acetic acid) and around 9.25 (pKa for ammonium). For this reason, ammonium acetate is commonly used as acetic acid to make buffer solutions. It is a volatile electrolyte that mimics the solubility properties of proteins under physiological conditions. Therefore, it is commonly used as a cell buffer for sample preparation in mass spectrometry.
Related articles And Qustion
Lastest Price from Ammonium acetate manufacturers
Ammonium acetate

US $8.80-1.10/kg2025-09-16
- CAS:
- 631-61-8
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Metal impurity≤ 100ppb)
- Supply Ability:
- 500kg
Ammonium Acetate

US $1200.00-1100.00/ton2025-08-08
- CAS:
- 631-61-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M