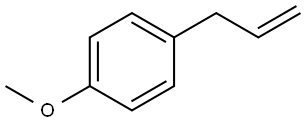
The toxicity of Estragole (4-Allylanisole)
Description
Estragole [4-(2-propenyl)-1-methoxybenzene] was nominated by the National Institute of Environmental Health Sciences (NIEHS) based on limited carcinogenicity studies in nursing mice following subcutaneous (s.c.) injection, which resulted in a significant increase in hepatocellular carcinomas. It is also structurally similar to the known carcinogen safrole [4-(2-propenyl)-1,2-methylenedioxybenzene] and has widespread use in food and as a fragrance. Estragole is listed on the U.S. Environmental Protection Agency High Production Volume Chemicals list with an estimated annual production volume of 2.8 to 3.8 million pounds (1,300 to 1,700 metric tons)[1].
Uses
Estragole is a phenylpropanoid chavicol, and the hydroxy group is replaced by a methoxy group. It has a role as a flavouring agent, an insect attractant, a plant metabolite, a genotoxin and a carcinogenic agent. It is an alkenylbenzene, a monomethoxybenzene and a phenylpropanoid.
Metabolized
Estragole is known to be metabolized along several pathways including O-demethylation (to give chavicol), epoxidation of the double bond, 1′-hydroxilation, and oxidative degradation of the side chain to carboxylic acids. Zangouras et al. indicate that at least two pathways, namely, O-demethylation and 1′-hydroxylation, exhibit dose-dependency in both mice and rats. Thus, the proportion of the dose that undergoes O-demethylation declines in a dose-dependent fashion and is accompanied by an increase in the dose that undergoes urinary elimination. This change presumably arises from the saturation of the enzyme systems responsible for O-dealkylation. The corollary of this is that ,a relatively greater substrate level would be available at higher doses for alternative metabolic reactions such as 1′-hydroxylation. In the mouse the major route of estragole metabolism is via hydroxilation at the 1′ position; producing derivatives with increased carcinogenic potential. Sulfuric acid esters of these compounds have been strongly implicated as the major ultimate electrophilic and carcinogenic metabolites in vivo. Thus, mouse liver cytosols contain 3′-phosphoadenosine 5′-phosphosulfate-dependent sulfotransferase activity for 1′-hydroxysafrole and 1′-hydroxydehydroestragole.
Estragole is metabolized via two major pathways: O-demethylation and 1’ hydroxylation. In humans, 58% of an oral dose was excreted in the urine in 48 hours, and 12% was exhaled as CO2 in 8 hours. In CD-1 mice, 23% of an intraperitoneal (i.p.) dose was excreted as 1'-hydroxyestragole as the glucuronide conjugate. In rodents, O-demethylation and 1'-hydroxylation are dose dependent, with O-demethylation the major pathway at low doses and 1'-hydroxylation the major pathway at higher doses.
Toxicity

Estragole is a natural constituent of basil oil. Several studies with oral, intraperitoneal or subcutaneous administration to CD-1 and B6C3F1 mice have shown that Estragole is carcinogenic. The 1-hydroxy metabolites are stronger hepatocarcinogens than the parent compound. Controversial results are reported for the mutagenicity of Estragole. However, the formation of hepatic DNA adducts in vivo and in vitro by metabolites of Estragole has been demonstrated.
Acute toxicity values (LD50) of about 1000 to 2000 mg/kg have been determined in the mouse and rat via i.p. and oral routes. Full-strength application of Estragole to the intact or abraded skin of rabbits was moderately irritating, but the dermal toxicity was low (LD50 >5000 mg/kg). In partially hepatectomized rats, Estragole significantly increased liver regeneration.
Carcinogenicity
Nursing CD-1 mice given three s.c. doses of Estragole developed hepatocellular carcinomas (i.e., malignant hepatomas). Estragole induced hepatomas [note: term used by the authors; unspecified whether malignant or benign] in preweanling and 8-week-old CD-1 mice dosed i.p. or orally or when fed in the diet. In B6C3F1 mice, Estragole induced hepatomas within 18 months in 83% of males given three doses as nursing pups and in 95% of male mice in 10 months following a single i.p. injection on day 12 of age. Of the metabolites identified in rodents and humans, only 1'-hydroxyestragole has been tested for carcinogenicity. Given s.c. to newborn CD-1 mice, hepatocellular carcinomas were induced by 12 months. Given i.p. or in the diet of mice, it induced hepatomas; susceptibility to hepatoma induction was influenced by strain, sex, and age. Rats treated s.c. for 10 weeks did not have an increased incidence of hepatic carcinomas.
References:
[1] L GORI. Can estragole in fennel seed decoctions really be considered a danger for human health? A fennel safety update.[J]. Evidence-based Complementary and Alternative Medicine, 2012. DOI:10.1155/2012/860542.You may like
See also
Lastest Price from Estragole manufacturers

US $1.20/kg2025-04-21
- CAS:
- 140-67-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100000

US $10.00/KG2025-04-21
- CAS:
- 140-67-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt