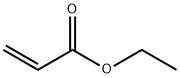
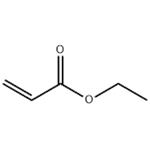
The Toxicity and Hazard of Ethyl acrylate
Ethyl acrylate is a colorless liquid,slightly soluble in water, ethanol and ether. It is produced by the action of 3-hydroxypropionitrile with ethanol and sulfuric acid, or the esterification of acrylic acid with ethanol. Ethyl acrylate monomer is used to make acrylic resins as well as emulsion (water-based) and solution (solvent-based) polymers. Water-based ethyl acrylate polymers are used in latex paints, caulks, leather finishing base coats, floor polishes, and textile finishes. Solvent-based ethyl acrylate polymers are used in lacquers, enamels, and lubricating oils.

Polymerization
Ethyl acrylate is a chemical intermediate manufactured and processed almost entirely within closed systems to produce homopolymers and copolymers with little remaining monomer (<0.1 wt%). Ethyl acrylate readily polymerizes upon standing and typically contains inhibitors to prevent spontaneous polymerization during storage. Ethyl acrylate is a volatile component of pineapple and Beaufort cheese.
Toxicity and Hazard Exposure Contents
Ethyl acrylate is a colorless liquid with a pungent pungent odor. It is irritating to the respiratory tract, high-concentration inhalation can cause pulmonary edema, direct eye contact can cause burns, and has obvious irritation and sensitization to the skin. Oral administration strongly stimulates the mouth and digestive tract, causing dizziness, difficulty breathing, and nervousness.
The toxic mode of action for ethyl acrylate is unknown. However, the parent compound may play a significant role since pretreatment of rats with a carboxylesterase inhibitor enhances the respiratory irritation and lethality produced by the inhalation of ethyl acrylate. The enhanced toxicity could be a direct effect of methyl acrylate on surrounding tissues and/or a secondary effect due to the increased conjugation of methyl acrylate with glutathione that occurs under these conditions which in turn can result in toxicity due to the depletion of local glutathione stores.
Ethyl acrylate is a volatile (40 hPa at 20 ℃) liquid under normal environmental conditions. At equilibrium in the environment, ethyl acrylate will partition primarily to air (88%) with the balance to water (12%). In air, ethyl acrylate will be removed by reaction with photochemically produced hydroxyl radicals (35.4 h half-life) and ozone (6.5 days halflife). In water, ethyl acrylate is relatively stable to hydrolysis at acidic and neutral pHs, but will volatilize to air (Henry’s law constant of 12.5 Pam-3 mol-1) or be biodegraded (57–90% removal in 28 days). Based on its relatively low octanol–water partition coefficient (log Kow of 1.18) and rapid metabolism in biological systems, ethyl acrylate does not pose a significant bioaccumulation hazard.
See also
Lastest Price from Ethyl acrylate manufacturers
US $0.00/kg2025-04-15
- CAS:
- 140-88-5
- Min. Order:
- 20kg
- Purity:
- 99.5%
- Supply Ability:
- 20 tons
US $100.00/KG2025-03-07
- CAS:
- 140-88-5
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 50tons