3-Hydroxypyrazine-2-carboxamide——Indication, Mechanism of action, Preparation etc.
General Description
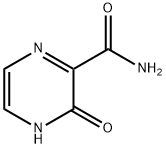
The 3-Hydroxypyrazine-2-carboxamide, with the CAS No: 55321-99-8, is also known as 3-oxo-3,4-dihydropyrazine-2-carboxamide, 2-Oxo-1H-pyrazine-3-carboxamide, 3-Hydroxy-2-pyrazinecarboxamide, T-1105. It belongs to the intermediate categories of Intermediates & Fine Chemicals; Pharmaceuticals; Amines; Aromatics; Heterocycles. This chemical’s molecular formula is C5 H5 N3 O2 and molecular weight is 139.11. It is a broad-spectrum viral polymerase inhibitor, a structural analogue of T-705, that inhibits the polymerase of RNA viruses after conversion to a ribonucleoside triphosphate (RTP) metabolite. T-1105 and T-705 are antiviral RNA nucleobase analogs that selectively inhibit the RNA-dependent RNA polymerase. They are expected as a drug candidate against various viral infections, including COVID-19 (1). 3-Hydroxypyrazine-2-carboxamide exhibits antiviral activity against a variety of RNA viruses, including Zika virus (ZIKV), influenza virus, sandy virus, bunyavirus, West Nile virus (WNV), yellow fever virus (YFV), and foot-and-mouth disease virus (FMDV).
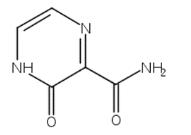
Figure 1 the molecular formula of 3-Hydroxypyrazine-2-carboxamide
Indication 
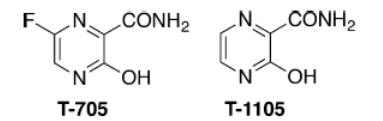
Figure 2 Chemical structures of T-705 and T-1105.
T-1105, a structural analogue of T-705 (favipiravir) has been reported to exert broad-spectrum antiviral effects against some RNA virus, including foot-and-mouth disease virus and bovine viral diarrhea virus (2). T-705, a nucleoside analogue that was first reported in 2002 (3), exerts potent broad-spectrum antiviral effects against many viruses, including influenza A, B, and C viruses, human and avian viruses, and even Ebola virus (in 2014) (4-5). This drug is also active against a wide variety of unrelated RNA viruses (reviewed by Furuta and coworkers) (6). With its unique mechanism of action and broad range of antiviral activity, favipiravir is a promising drug candidate for influenza and many other RNA viral diseases for which there are no approved therapies (6).
The GP model was validated by demonstrating the antiviral effect of T-1105 (an influenza virus inhibitor with reported activity against FMDV). Mean viral RNA levels in serum and organs of T-1105-treated and vaccinated animals were reduced compared to untreated controls. T-1105 conferred a substantial clin. and virol. protection against infection with O1 Manisa, similar to the protection afforded by vaccination. It validates the suitability of the enhanced GP model for the purpose of initial evaluation of inhibitors of FMDV replication and illustrate the potential of selective inhibitors of viral replication to control FMD (7).
The therapeutic agent comprises a combination of a pyrazine deriv. or a salt thereof, and a compd. which increases the amt. of pyrazine deriv. ribose triphosphate bodies in cells, exhibits an effect against an RNA virus, and can simultaneously potentiate antiviral activity against a plurality of RNA viruses. Examples of the pyrazine deriv. include 6-Fluoro-3-hydroxy-2-pyrazinecarboxamide (T-705) and T-1105, and the combination compd. which increases the amt. of intracellular pyrazine deriv. ribose triphosphate includes antifolate, thiopurine-based antimetabolic agent, tiazofurin, alkylating agent and xanthine deriv. The therapeutic agent may further contain another anti-RNA virus agent, e.g. ribavirin (8).
Mechanism of action
T-1105 and T-705 are antiviral RNA nucleobase analogs that selectively inhibit the RNA-dependent RNA polymerase (1). Favipiravir is a prodrug that is phosphoribosylated in cells to the active form, favipiravir-RTP, which inhibits influenza viral replication. Thus, favipiravir may be misincorporated in nascent viral RNA, or it may act by binding to conserved polymerase domains, preventing incorporation of nucleotides for viral RNA replication and transcription. (6)
Toxicity
The most common adverse effects include low cytotoxicity (6).
The antiviral drug T-705 (favipiravir) and its non-fluorinated analog T-1105 inhibit the polymerases of RNA viruses after being converted to their ribonucleoside triphosphate (RTP) metabolite. The activation efficiency of T-705 and T-1105 in four cell lines that are commonly used for their antiviral evaluation were compared. In MDCK cells, the levels of T-705-RTP were markedly lower than those of T-1105-RTP, while the opposite was seen in A549, Vero and HEK293T cells. In the latter three cell lines, T-1105 activation was hindered by inefficient conversion of the ribonucleoside monophosphate to the ribonucleoside diphosphate en route to forming the active triphosphate. Accordingly, T-1105 had better anti-RNA virus activity in MDCK cells, while T-705 was more potent in the other three cell lines. It identified a fourth metabolite, the NAD analog of T-705/T-1105, and showed that it can be formed by NMN adenylyltransferase (9).
Preparation
To a suspension of compound 2-aminomalonamide in water glyoxal was added. The reaction mixture was stirred at 90 °C for 3 h, then cooled down to room temperature. After neutralization with 15% NH4OH solution until pH > 7, H2O2 was added dropwise at 0 °C and the reaction mixture was stirred at room temperature for 1 h. The precipitate was filtered, washed with acetone and crystallized in water to give the desired pure pyrazine compound. m.p. 269–271 °C. (10)
An alternative process in Jpn Jpn. Kokai Tokkyo Koho, 2010241806 involves that sodium hydroxide and 85% phosphoric acid were mixed with water and a phosphate buffer solution was obtained. To the phosphate buffer solution, amino malonic amide was added at 20~30 °C, water solution of sodium hydroxide and glyoxal aqueous solution were dropped simultaneously over 1 hour and was stirred at this temperature for 30 minutes. To the reaction mixture, concentrated hydrochloric acid was added, heated to 85 °C, concentrated hydrochloric acid was added and cooled to 15 °C. The solid was filtered off and 3-hydroxy-2-pyrazine carboxamide was obtained as a brown solid. (11)
References
1. Nakayama, Tatsushi; Honda, Ryo. Electrochemical and mechanistic study of oxidative degradation of favipiravir by electrogenerated superoxide through proton-coupled electron transfer. ACS Omega 2021, 6(33), 21730-21740.
2. Florescu DF, Kalil AC, Hewlett AL, Schuh AJ, Stroher U et al.) Administration of brincidofovir and convalescent plasma in a patient with ebola virus disease. Clin Infect Dis. 2015, 61:969–973
3. Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002, 46:977–981
4. Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 2005,49:981–986
5. 20. Oestereich L, Ludtke A, Wurr S, Rieger T, Munoz-Fontela C et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014, 105:17–21
6. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013, 0:446–454.
7. De Vleeschauwer, A. R.; Lefebvre, D. J.; Willems, T.; Clercq, K. et al. A Refined Guinea Pig Model of Foot-and-Mouth Disease Virus Infection for Assessing the Efficacy of Antiviral Compounds. Transboundary and Emerging Diseases (2016), 63(2): 205-212.
8. PCT Int. Appl. (2020), WO 2020138067 A1 20200702
9. Huchting, Johanna; Vanderlinden, Evelien; Van Berwaer, Ria, et al. Cell line-dependent activation and antiviral activity of T-1105, the non-fluorinated analogue of T-705 (favipiravir). Antiviral Research 2019, 167, 1-5.
10. Pierra, Claire et al. Synthesis and antiviral evaluation of the 2'-Cmethyl branched derivative of a nucleoside analog inhibitor of RNA viral infections, T-1106. Collection of Czechoslovak Chemical Communications, 2011,76(11), 1327-1333;
11. Jpn. Kokai Tokkyo Koho, 2010241806
You may like
Related articles And Qustion
Lastest Price from 3-HYDROXYPYRAZINE-2-CARBOXAMIDE manufacturers

US $10.00/KG2021-08-31
- CAS:
- 55321-99-8
- Min. Order:
- 25Kg/Drum
- Purity:
- 99.99%
- Supply Ability:
- 20 tons/month

US $0.00-0.00/Kg2020-04-30
- CAS:
- 55321-99-8
- Min. Order:
- 1KG
- Purity:
- 99.0%+
- Supply Ability:
- 800 tons