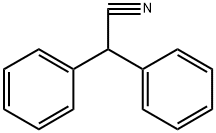
The synthetic methods of diphenylacetonitrile
General description
The molecular formula of diphenylacetonitrile is C14H11N and the molecular weight is 193.25. In medicine, it is used to produce gastric amine, phenethylpiperidine, and other drugs. It can be used to control grass young grass in turf before bud. Generally, it can be obtained from phenylacetonitrile by bromination and condensation.
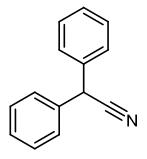
Fig. 1 The structure of Diphenylacetonitrile.
Physicochemical property
Diphenylacetonitrile is a white crystal with melting point of 76℃ and boiling point of 181℃ (1.6kPa). It is soluble in ethanol and ether.
Synthetic routes
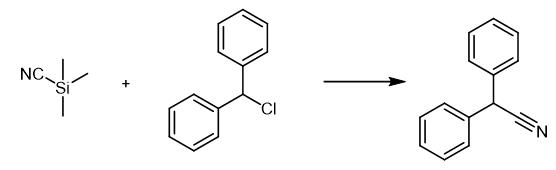
Fig. 2 The synthetic scheme 1 of Diphenylacetonitrile.
Charge a round-bottomed flask with allylic alcohol (0.3 mmol) in DCM (5 mL), Li2CO3 (0.06 mmol), TMSCN (1.35 mmol) and I2 (0.54 mmol) in sequence successively. Stir the resulting mixture under closed conditions at 35 °C (water bath temperature) for 5 hours. Quench the reaction with saturated solution of Na2S2O3. Separate the organic phase. Extract the aqueous layer with DCM (5 mL × 3). Dry the combined organic solution with Mg2SO4. Concentrate the combined organic solution in vacuo. Purify the resulting residue by a column chromatography. 1H NMR (500 MHz, CDCl3) δ 7.39-7.29 (m, 10H), 5.13 (s, 1H). 13C NMR (125 MHz, CDCl3) δ 136.0, 129.3, 128.4, 127.8, 119.8, 42.7 [1].
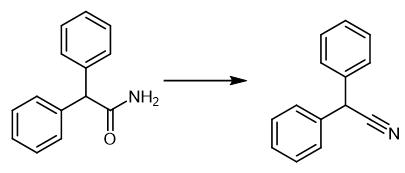
Fig. 3 The synthetic scheme 2 of Diphenylacetonitrile.
Cool an oven-dried 100 mL one-neck round-bottom flask to room temperature under a stream of dry nitrogen gas, supplied by an inert gas manifold. Charge the flask with 2,2-diphenylacetamide (5.28 g), copper(II) acetate (45.4 mg, 0.25 mmol, 0.01 equivalent), bis(dicyclohexylphosphino)ethane (0.011 mg, 0.275 mmol, 0.011 equivalents) and a magnetic stirring bar. Seal the flask with a rubber septum. Insert a needle connected to the inert gas manifold through the septum. Place the flask under vacuum. Refill the flask with dry nitrogen. Repeat the process a total of three times. Leave the flask under a slight positive pressure of nitrogen supplied through the manifold. Add THF (25 mL) to the mixture. Stir the mixture for 5 minutes until a deep blue colored solution formed. Add poly(methylhydrosiloxane) (6.0 mL, 100 mmol hydride, 4 equivalents) slowly to the mixture using a 12 mL plastic syringe with a needle attached over 5 minutes. Allow the reaction mixture to stir at room temperature for 12 hours. Remove the septum carefully. Quench the reaction by slowly pouring into a 500 mL Erlenmeyer flask containing saturated ammonium fluoride in methanol (200 mL). Stir the mixture for 30 minutes at room temperature. Concentrate the mixture with the aid of a rotary evaporator. Suspend the residue in EtOAc (300 mL). Filter the residue through a short plug of Celite (~200 g) with additional EtOAc (300 mL). Wash the combined filtrate sequentially with water (2 × 200 mL) and brine (200 mL). Dry the combined filtrate over sodium sulfate. Subject the combined filtrate to gravity filtration. Concentrate the filtrate with the aid of a rotary evaporator. Purify the crude product by recrystallization from hot acetone. 1H NMR (400 MHz, CDCl3): δ 7.41-7.32 (m, 10H), 5.15 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 136.0, 129.3, 128.4, 127.9, 119.8, 42.7 [2].
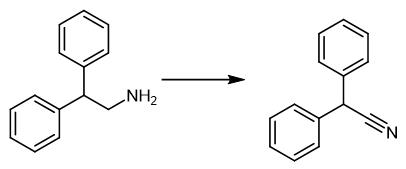
Fig. 4 The synthetic scheme 3 of Diphenylacetonitrile.
Place corresponding amine (0.5 mmo) and a magnetic stirring bar in a glass vial (7 mL). Add t-amyl alcohol (4 ml), Fe2O3/NGr@C (40 mg) followed by of aqueous NH3 (100-200 μl) to the mixture. Fit the vial with septum, cap, and needle. Place the reaction vial into a 300 mL autoclave. Pressurize the autoclave with 3 bar of oxygen. Place the autoclave into an aluminum block (place 30 minutes before counting the reaction time in order to attain reaction temperature). Preheat the autoclave at 120 °C. Stir the reaction for 15 hours at 110°C. Cool the autoclave to room temperature, after completion of the reaction. Discharge the remaining oxygen. Remove the sample from the autoclave. Filter the mixture through a plug of silica. Wash the mixture with ethyl acetate. Remove the solvent from the filtrate in vacuo. Purify the residue by column chromatography (silica; nhexane/ethyl acetate mixture) to obtain the product [3].
Application
Aggregation enhanced emission (AIE) effects
A novel diphenylacrylonitrile derivative (Z)-3-(4'-(diphenylamino)-[1,1'-biphenyl]-4-yl)-2-(4-methoxyphenyl)-acrylonitrile (beta-CN-TPA) containing a twisted triphenylamine and diphenylacetonitrile was synthesized via Knoevenagel condensation and Suzuki coupling reactions. These molecules exhibited aggregation enhanced emission (AIE) effects. Interestingly, their mechano-fluorochromic properties were invisible upon grinding with a pestle. However, when hydrostatic pressure in a diamond anvil cell (DAC) was applied on the crystals of beta-CN-TPA, the distinct piezochromic behaviors of the compound were observed. The fluorescence color changed from light green (530 nm) to red (665 nm) with a significant red-shift of 135 nm. The powder X-ray diffraction and high-pressure Raman studies indicated that the as-synthesized and ground samples had the same crystalline structures, while the compressed samples had an evident change in inter-molecular interactions. Comparative tests and theoretical analysis further confirmed that the distinct fluorescence behaviors of the desired dye during the different stress conditions were associated with the various inter-molecular interactions that existed with adjacent molecules [4].
References
[1] Hu L, Hussain M I, Deng Q, et al. I2/Li2CO3-promoted cyanation of diarylalcohols through a dual activation process[J]. Tetrahedron, 2019, 75(2): 308-314.
[2] Liu R Y, Bae M, Buchwald S L. Mechanistic insight facilitates discovery of a mild and efficient copper-catalyzed dehydration of primary amides to nitriles using hydrosilanes[J]. Journal of the American Chemical Society, 2018, 140(5): 1627-1631.
[3] Jagadeesh R V, Junge H, Beller M. “Nanorust”‐catalyzed Benign Oxidation of Amines for Selective Synthesis of Nitriles[J]. ChemSusChem, 2015, 8(1): 92-96.
[4] Ouyang M, Zhan L, Lv X, et al. Clear piezochromic behaviors of AIE-active organic powders under hydrostatic pressure[J]. RSC advances, 2016, 6(2): 1188-1193.
You may like
Related articles And Qustion
See also
Lastest Price from Diphenylacetonitrile manufacturers

US $10.00-1.00/kg2025-10-31
- CAS:
- 86-29-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 600tons

US $1.00/g2025-04-21
- CAS:
- 86-29-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg