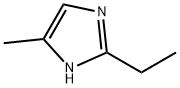
Synthesis and Application of 2-ethyl-4-methylimidazole
General description
2-ethyl-4-methylimidazole is soluble in epoxy resin. Reference dosage 2-7phr. 2-ethyl -4-methyl imidazole can be widely used in epoxy resin bonding, coating, casting, encapsulation, impregnation and composite materials. EMI-24 cured epoxy resin is better than m-phenylenediamine curing agent in heat resistance, oxidation resistance, drug resistance, especially acid resistance. The deformation resistance temperature of EMI-24 curing agent increases with the increase of its dosage and curing temperature.
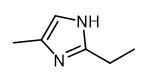
Fig. 1 The structure of 2-ethyl-4-methylimidazole.
Physicochemical property
2-ethyl-4-methylimidazole is a light yellow crystal with a melting point of 45℃. It has a boiling point of 292-295℃, a relative density of 0.975 at 45℃, a refractive index of 1.4995, and a flash point of 137℃. At 20℃, its solubility in water is 210 g/L.
Synthetic routes
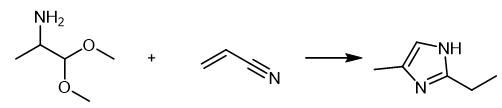
Fig. 2 The synthetic scheme 1 of 2-ethyl-4-methylimidazole.
185 g of 2-aminopropanal dimethyl alcohol (1.56 mol) was added into a three-way flask, followed by 106 g of propanonitrile (1.95 mol) and 279 g of cuprous bromide (1.95 mol). The mixture was stirred and heated to 85°C, kept warm for 8 hours, and then stopped stirring and cooled to room temperature. 1200 ml of methanol and 380 g of concentrated hydrochloric acid (37% by mass) were added, and methanol was decompressed at 60°C for 4 hours. 500 g of sodium hydroxide (50% by mass) was added in an ice bath at a holding temperature not exceeding 20°C. After stirring for 15 minutes, 3000 ml of methyl tert-butyl ether was added. After stirring for 15 minutes, the solid body was washed with 500ml methyl tert-butyl ether, combined with organic phase and dried with anhydrous sodium sulfate. The solvent was decompressed and the solid product was 118.8g with a yield of 64.1% (calculated by 2-amino-propanal dimethyl alcohol) [1].
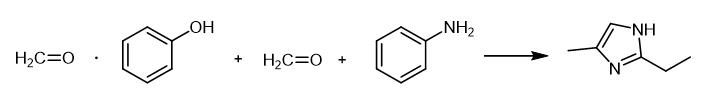
Fig. 3 The synthetic scheme 2 of 2-ethyl-4-methylimidazole.
Bisphenol A (27.39 g, 0.12 mole) and paraformaldehyde (14.40 g, 0.48 mole), and MEK were added to a 400-mL beaker. While stirring, aniline (22.37 g, 0.24 mole) was added and the mixture heated to 120° C using a hot plate. During the reaction, MEK and water were allowed to evaporate producing a yellow and viscous mass. The mixture was allowed to equilibrate to room temperature at which point it solidified. Polybenzoxazine (B7) [2].
Application
Cross-linking agent
A co-cross-linking reaction of bio-based multi-functional epoxides (1) derived from limonene oxide and bisphenol A diglycidyl ether (BPADE) in the presence of 2-ethyl-4-methylimidazole (EMI) as a cross-linker afforded the corresponding network copolymers (2) having the 10% thermal decomposition temperature (T-d10) of 294.4 degrees C at maximum. Adhesive strength induced by 1, especially tetra-functional epoxide, and SPADE exhibited remarkably higher than that by BPADE alone [3].
Epoxy curing agent
For the first time, repeatable self-healing was achieved in a cross-linked epoxy polymer by incorporating 2-ethyl-4-methylimidazole (24-EMI) into the matrix as a latent polymerization initiator. Upon material damage and infiltration of liquid EPON 8132 epoxy monomer healing agent into the crack plane, polymerization occurs in the damaged region with a moderate application of heat in the presence of the latent imidazole initiator. Using tapered double cantilever beam (TDCB) fracture testing, greater than 90% recovery of fracture toughness was observed over multiple healing cycles in samples containing 10 wt% 24-EMI, with up to 11 repeat healing cycles possible. The effect of incorporating the imidazole on the host epoxy fracture toughness, complex moduli and glass transition temperature was also investigated. As imidazole concentration increases, a reduction in glass transition temperature and an increase in fracture toughness of the host epoxy is observed [4].
The system of bisphenol-A diglycidyl ether type epoxy resin (DGEBA) cured with 2-ethyl-4-methylimidazole (2,4-EMI) was studied by means of differential scanning calorimetry (DSC) in isothermal mode. Analysis of DSC curves indicated that the curing reaction could be separated into two stages, the addition reaction and the catalytic polymerization, as proposed in the literatures. The catalytic polymerization reaction has an induction period which decreases with the increasing of curing temperature (T-c). The rate constants for the catalytic polymerization stage were calculated from DSC studies. A curing kinetic model separated into two steps by the induction period was developed based on the reaction mechanism. The model can predict part of the experimental results,but some deviations are observed at the higher values of curing degree (alpha). A critical value of curing degree (alpha(c)) and a diffusion factor were introduced, and a diffusion controlled curing kinetic model was developed. For the systems of various concentrations of 2,4-EMI cured in the temperature range of 90 similar to 120 degrees C, the values of alpha(c) were calculated. The studies show that the diffusion factors affect the curing kinetics greatly in the higher values of alpha. When the value of a is low, the reaction is controlled by the chemical factors, but it is controlled by diffusion, when alpha is high. The value of alpha(c) is mainly affected by the glass transition of the system. With the increase of T-c, the curing degree at the glass transition increases and thus the value of alpha(c) increases too [5].
The influence of surface treated multiwalled carbon nanotubes (MWCNTs) on the cure behavior of a bisphenol-A glycidol ether epoxy resin/2-ethyl-4-methylimidazole system was investigated with non-isothermal differential scanning calorimetry. With the increase of the MWCNT content, the initial curing temperature, exothermic peak temperature and cure reaction activation energy first increase and subsequently decrease. The addition of MWCNTs results in a continuous increase in the degree of vitrification in the epoxy system, so that the cure reaction is likely to be diffusion controlled at lower heating rate and larger conversion fractions [6].
References
[1] Qi G, Shen J, Shi A, et al. Method for preparing 2-ethyl-4-methylimidazole by one-pot synthesis [P]. Faming Zhuanli Shenqing, 103086977, 2013.
[2] Liang R-C, McNamara J J, Ying Y, Hou C-J. Phenolic-epoxy, phenolic-benzoxazine, phenolic-epoxy-benzoxazine curable adhesive compositions, their manufacture and applications [P]. PCT Int. Appl., 2009089314, 2009.
[3] Morinaga H, Sakamoto M. Co-cross-linking of bio-based multi-functional epoxide and bisphenol A diglycidyl ether with 2-ethyl-4-methylimidazole[J]. Tetrahedron Letters, 2018, 59(43): 3889-3891.
[4] Hart K R, Sottos N R, White S R. Repeatable self-healing of an epoxy matrix using imidazole initiated polymerization[J]. Polymer, 2015, 67: 174-184.
[5] Pang P J, Shan G R, Huang Z M, et al. Curing kinetic model of the 2-ethyl-4-methylimidazole/epoxy system[J]. ACTA POLYMERICA SINICA, 2006 (1): 21-25.
[6] Yang K, Gu M, Jin Y, et al. Influence of surface treated multi-walled carbon nanotubes on cure behavior of epoxy nanocomposites[J]. Composites Part A: Applied Science and Manufacturing, 2008, 39(10):
Related articles And Qustion
See also
Lastest Price from 2-Ethyl-4-methylimidazole manufacturers

US $3.00/kg2025-04-21
- CAS:
- 931-36-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $10.00/KG2025-04-21
- CAS:
- 931-36-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt