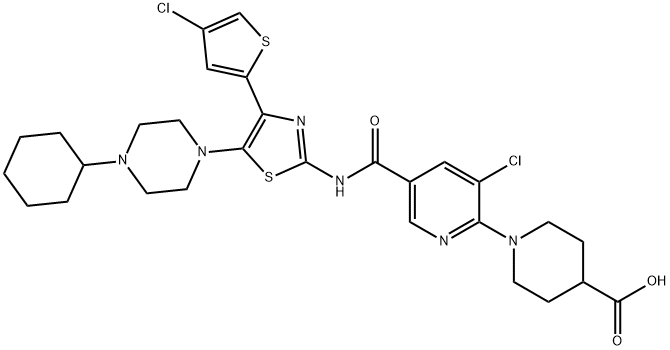
The synthesize method of Avatrombopag
Description
Avatrombopag (Doptelet®) is an orally administered second-generation TPO-RA approved in the USA and the EU for the treatment of primary chronic ITP in adult patients who are refractory to (EU) or have had insufficient response to (USA) other treatments the drug is also approved in the USA for the treatment of thrombocytopenia in adult patients with CLD who are scheduled to undergo a procedure, and in the EU for the treatment of severe thrombocytopenia in adult patients with CLD who are scheduled to undergo an invasive procedure[1].
Development
It is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. The USFDA first approved it in May 2018 and subsequently approved by the European Medicine Agency (EMA) in June 2019. Initially developed by Astellas, avatrombopag's development rights have been transferred between several firms, most recently Dova Pharmaceuticals (an affiliate of PBM Capital). In March 2018, Dova entered into an agreement (through AkaRx) to grant Shanghai Fosun Pharma the drug's exclusive development and distribution rights in mainland China and Hong Kong.
Synthesize method
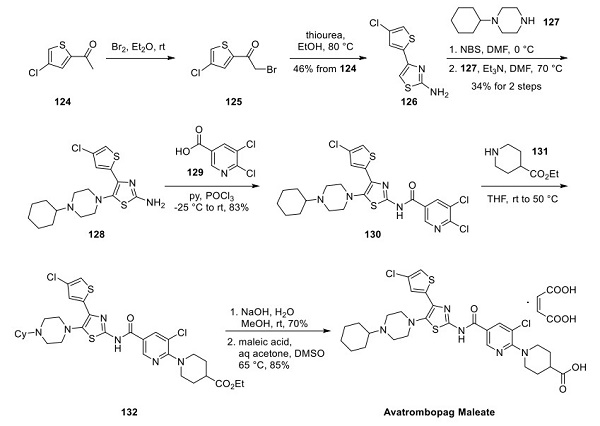
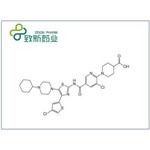
A large-scale synthetic route to avatrombopag and crystalline form protocols have been reported in a series of patents from Astellas[2]. As shown above, bromination of 1-(4-chlorothiophen-2-yl)ethenone (124) gave bromide 125. Condensation with thiourea produced thiazolamine 126 in 46% yield over two steps. Thiazolamine 126 was brominated with N-bromosuccinimide (NBS) in DMF, allowing nucleophilic aromatic substitution with 1-cyclohexylpiperazine (127) to provide 128 in 34% overall yield. Amide bond formation with 5,6-dichloro nicotinic acid (129) was accomplished by activation with phosphorus oxychloride to give nicotinamide 130 in 83% yield. A second nucleophilic aromatic substitution with ethyl isonipecotate 131, followed by hydrolysis, provided avatrombopag (132). Salt formation was demonstrated on 20 kg scale using maleic acid in a mixture of DMSO/acetone/water (2:2:1) to obtain avatrombopag maleate in 85% yield.
References
[1] Markham, Anthony. “Avatrombopag: A Review in Thrombocytopenia.” Drugs (2021): 1905–1913.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
Related articles And Qustion
See also
Lastest Price from Avatrombopag manufacturers

US $2.00-5.00/KG2025-07-10
- CAS:
- 570406-98-3
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons
US $0.00-0.00/kg2025-04-21
- CAS:
- 570406-98-3
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 5kg/Month