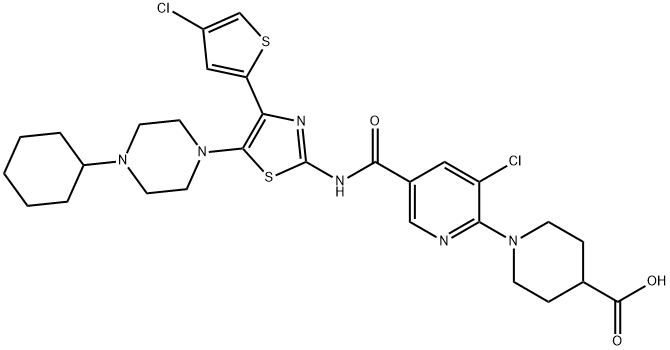
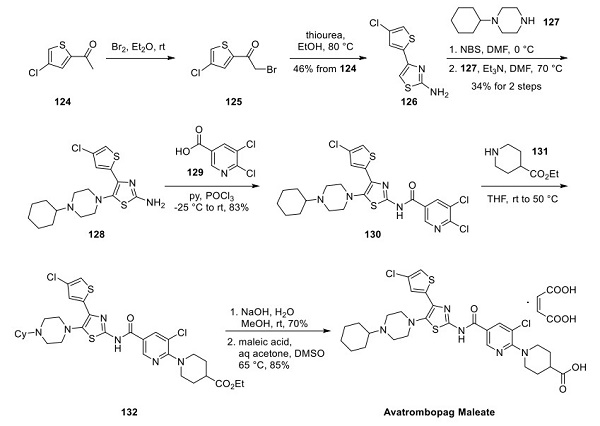
Avatrombopag: mechanism of action, pharmacokinetics and clinical applications
General Description
Avatrombopag is a medication used to treat thrombocytopenia, a condition characterized by low platelet count. It mimics the effect of thrombopoietin, the primary regulator of platelet production, by binding to the thrombopoietin receptor and activating signal transduction pathways. Avatrombopag promotes the proliferation and differentiation of platelet-producing cells, leading to an increase in platelet count. It is mainly metabolized by cytochrome P450 enzymes and excreted in feces. Avatrombopag is clinically used to raise platelet counts in patients with chronic liver disease undergoing medical procedures and in those with immune thrombocytopenia who are unresponsive to other treatments. It offers a valuable therapeutic option for thrombocytopenia patients but should be used under healthcare professional guidance.
Figure 1. Tablets of avatrombopag
Mechanism of action
Avatrombopag is a medication used to treat thrombocytopenia, a condition in which the body has a low platelet count. The drug functions by mimicking the effect of thrombopoietin, the primary regulator of platelet production in the body. Thrombopoietin's receptor is expressed on various haematopoietic cells, including stem cells, megakaryocyte colony forming cells, and mature platelets. Upon activation of the receptor, several signal transduction pathways are initiated, notably JAK/STAT, MAPK, and various anti-apoptotic pathways. Avatrombopag was found to induce proliferation of human thrombopoietin receptor-expressing murine Ba/F3 cells in a concentration-dependent manner, with maximal activity similar to that of recombinant human thrombopoietin. It also promoted megakaryocyte differentiation from human cord blood CD34+ cells, and the proliferation of haematopoietic and megakaryocytic progenitor cells. In non-obese diabetic/severe combined immunodeficiency mice transplanted with human fetal liver CD34+ cells, oral administration of avatrombopag produced a dose-dependent increase in human platelet count. Notably, the drug binds to a different site on the thrombopoietin receptor than the endogenous ligand, and treatment with avatrombopag was not associated with an increase in platelet activation or platelet reactivity in patients with CLD undergoing treatment. 1
Pharmacokinetics
After oral administration, avatrombopag is absorbed slowly with a short lag time of 0.5-0.75 hours and reaches maximum plasma concentration after 6-8 hours. Steady state is achieved by day 5 in multiple-dose studies. The exposure to avatrombopag increases proportionally to the dose up to 80 mg. The presence of high or low-fat food does not significantly affect the maximum plasma concentrations (Cmax) or the area under the plasma concentration-time curve (AUC). The drug is mainly metabolized by cytochrome P450 enzymes, specifically CYP2C9 and CYP3A. Most of the unchanged drug and its metabolites are excreted in the feces (88%), with no detectable metabolites in plasma. The terminal half-life is approximately 19 hours. When avatrombopag was coadministered with fluconazole, a strong inhibitor of CYP2C9 and CYP3A, there was a twofold increase in avatrombopag exposure and doubling of the half-life. This led to a clinically relevant 1.66-fold increase in platelet count. A population pharmacokinetic analysis showed that avatrombopag follows a one-compartment model with simultaneous first- and zero-order absorption, and linear elimination. Bodyweight and the presence of CLD affect the apparent volume of distribution. East Asian ethnicity, as well as increasing albumin and thrombopoietin levels, reduce the effect of avatrombopag on platelet production, although these effects are not considered clinically relevant. 2
Clinical applications
Avatrombopag is a medication with various clinical applications. It is primarily used for the treatment of thrombocytopenia in patients with chronic liver disease (CLD) who are scheduled to undergo a medical procedure. Thrombocytopenia refers to a low platelet count, which can result in an increased risk of bleeding. In clinical trials, avatrombopag has demonstrated its effectiveness in raising platelet counts and reducing the need for platelet transfusions in patients with CLD-associated thrombocytopenia. By stimulating the production and maturation of platelets, avatrombopag helps restore platelet levels to a safe range, thus minimizing the risk of bleeding complications during medical procedures. Additionally, avatrombopag has shown promise in the treatment of immune thrombocytopenia (ITP), a condition characterized by low platelet counts due to the body's immune system mistakenly attacking its own platelets. In patients with ITP who have not responded well to other treatments, avatrombopag has been found to increase platelet counts and reduce the need for concomitant therapies. The clinical application of avatrombopag offers a valuable therapeutic option for patients with thrombocytopenia, both in the setting of CLD and ITP. However, it is important to note that avatrombopag should always be used under the guidance of a healthcare professional, as individual patient factors and potential drug interactions need to be considered to ensure safe and effective. 3
Reference
1. Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013, 98(1):10–23.
2. Swedish Orphan Biovitrum. Doptelet® (avatrombopag): EU summary of product characteristics. 2021.
3. Kuter DJ. The structure, function, and clinical use of the thrombopoietin receptor agonist avatrombopag. Blood Rev. 2022, 53:100909.
Related articles And Qustion
See also
Lastest Price from Avatrombopag manufacturers

US $2.00-5.00/KG2025-07-10
- CAS:
- 570406-98-3
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons
US $0.00-0.00/kg2025-04-21
- CAS:
- 570406-98-3
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 5kg/Month