Avatrombopag: Pharmacodynamic Properties, Therapeutic Efficacy, Dosage and Administration
General Description
Avatrombopag, a thrombopoietin receptor agonist, mimics endogenous thrombopoietin to stimulate platelet production by promoting megakaryocyte growth and differentiation. Avatrombopag exhibits targeted effects on platelet production without affecting platelet function, making it a promising therapy for immune thrombocytopenia (ITP) and chronic liver disease (CLD)-associated thrombocytopenia. Clinical trials of Avatrombopag have demonstrated its efficacy in increasing platelet counts and reducing the need for rescue therapy in both ITP and CLD patients before elective procedures. The recommended dosing varies for each condition, emphasizing regular platelet monitoring and cautious dose adjustments to maintain stable platelet levels.

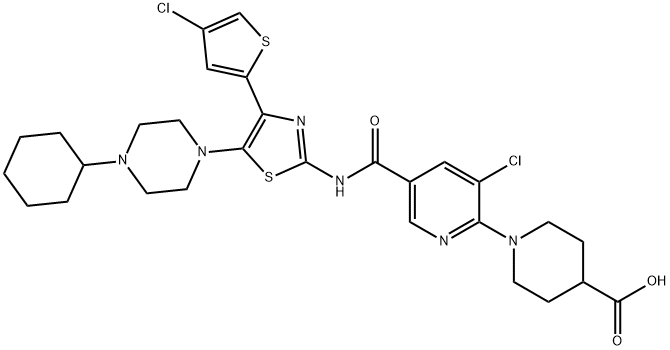
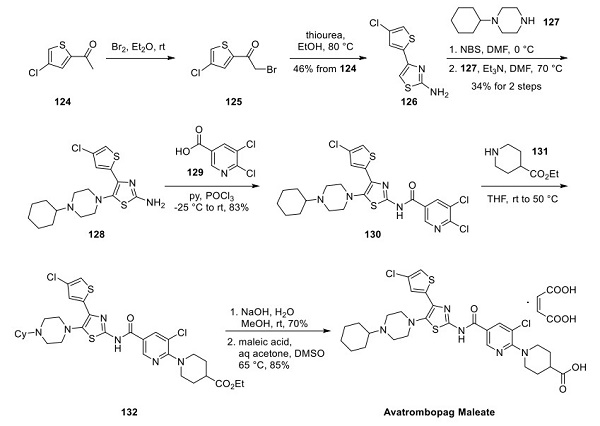
Figure 1. Avatrombopag
Pharmacodynamic Properties
Avatrombopag exerts its pharmacodynamic effects by mimicking the actions of the endogenous ligand thrombopoietin, which plays a crucial role in regulating platelet production through the stimulation of megakaryocyte growth. Thrombopoietin binds to its receptor, activating various signal transduction pathways such as JAK/STAT, MAPK, and anti-apoptotic pathways. In vitro studies have shown that avatrombopag induces the proliferation of human thrombopoietin receptor-expressing cells in a concentration-dependent manner, resulting in the phosphorylation of STAT3, STAT5, and MAPK (ERK). It also promotes megakaryocyte differentiation from human cord blood cells and enhances megakaryocyte proliferation when combined with recombinant human thrombopoietin. Moreover, avatrombopag increases human platelet count in mice models transplanted with human fetal liver cells in a dose-dependent manner. Notably, in patients with Chronic Liver Disease avatrombopag treatment has been shown to elevate platelet counts without increasing platelet activation or reactivity, indicating its specific and targeted action on platelet production without affecting platelet function. This unique pharmacodynamic profile of avatrombopag highlights its potential as a therapeutic agent for conditions characterized by low platelet counts, such as immune thrombocytopenia and CLD. 1
Therapeutic Efficacy
Avatrombopag has shown promising therapeutic efficacy in the treatment of chronic immune thrombocytopenia and thrombocytopenia associated with chronic liver disease prior to elective procedures. In chronic ITP, two phase III trials were conducted to evaluate the efficacy of avatrombopag. In a placebo-controlled study, avatrombopag demonstrated superiority over placebo in terms of the cumulative mean number of weeks of platelet response without requiring rescue therapy. A significant percentage of avatrombopag recipients achieved a platelet response at day 8 compared to the placebo group. Additionally, durable responses were observed in a notable proportion of avatrombopag recipients. In the context of thrombocytopenia associated with CLD, the phase III ADAPT program showcased the effectiveness of avatrombopag in reducing the need for platelet transfusions or rescue procedures before elective interventions. Avatrombopag outperformed placebo in both low and high baseline platelet count cohorts, with a substantial number of avatrombopag recipients achieving the primary endpoint compared to placebo recipients. The integrated analysis of pooled data further confirmed the significant efficacy of avatrombopag, emphasizing its ability to maintain target platelet counts and reduce the requirement for interventions across different risk categories of procedures. Overall, avatrombopag presents as a promising therapeutic option for managing chronic ITP and CLD-associated thrombocytopenia, demonstrating consistent efficacy in improving platelet counts and reducing the need for additional interventions in these patient populations. 1
Dosage and Administration
Avatrombopag is dosed differently based on the condition being treated. In patients with Immune Thrombocytopenia, the initial dose is 20 mg once daily, which can be adjusted to maintain a stable platelet count between ≥ 50 and ≤ 150 × 10^9/L. Platelet levels should be checked weekly. For patients with Chronic Liver Disease, the recommended dose is 60 mg once daily if the platelet count is < 40 × 10^9/L, or 40 mg once daily if the count is between 40 and < 50 × 10^9/L. This should be taken for 5 days, starting 10-13 days before a planned procedure, with the last dose administered 5-8 days before the procedure. Platelet counts must be checked before giving avatrombopag and on the day of the procedure to confirm an adequate increase. Monitoring for thrombocytosis is crucial as platelet counts may rise rapidly. Once a stable platelet count is reached, monthly monitoring is recommended. After stopping avatrombopag, weekly platelet counts are needed for at least 4 weeks. It's essential to follow local prescribing information for detailed guidelines on dosage adjustments, monitoring, warnings, and special population considerations. Do not aim to normalize platelet counts with avatrombopag in ITP or CLD patients. 2
Reference
1. Markham A. Avatrombopag: A Review in Thrombocytopenia. Drugs. 2021 Nov;81(16):1905-1913. doi: 10.1007/s40265-021-01613-y. Epub 2021 Oct 28. Erratum in: Drugs. 2021 Dec;81(18):2169.
2. Swedish Orphan Biovitrum. Doptelet (avatrombopag): EU summary of product characteristics. 2021.
You may like
Related articles And Qustion
See also
Lastest Price from Avatrombopag manufacturers

US $2.00-5.00/KG2025-07-10
- CAS:
- 570406-98-3
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons
US $0.00-0.00/kg2025-04-21
- CAS:
- 570406-98-3
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 5kg/Month