Is CH3Cl Polar or Nonpolar?
CH3Cl is the chemical formula for the organic compound chloromethane, also known as methyl chloride. It is a clear, colorless gas and has a faintly sweet odor. CH3Cl has a molar mass of 50.49 g/mol and is used as an extractant for oil and resins, a propellant in foam production, etc. It is also used as a solvent in rubber production and in petroleum refining.
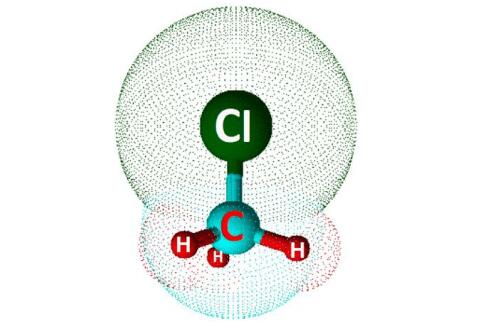
Polarity of CH3Cl
Chloromethane, having a chemical formula CH3Cl, has one atom of Carbon in its central position, three hydrogen atoms, and one atom of Chlorine. As Carbon is less electronegative than Chlorine, it takes a central position, and rest all atoms are arranged around it.
When you look at the Lewis dot structure of this molecule, there are no lone pairs of electrons in its structure. Carbon shares its four electrons, which completes the octet of all the atoms in the molecule. In total, there are 14 valence electrons of all the three atoms that participate in forming bonds giving a tetrahedral shape to the molecule.
Suppose you compare the electronegativities of both the atoms carbon and Chlorine. In that case, you will find out that the chlorine atom is more electronegative than the carbon atom as it is closer to Flouirne on the periodic table. So there will be a dipole moment between Chlorine and Carbon atom. As Chlorine has more electronegativity, it tries to pull the electrons on its side. This dipole moment results in the unequal distribution of charges on Carbon and Chlorine. Thus C-Cl bond is considered to be polar.
When it comes to the bond between Carbon and Hydrogen atoms, the difference in the electronegativities of both these atoms is relatively small. Due to which the C-H bond is considered nonpolar.
However, as there are partial negative charges on the Chlorine atom and have a net dipole moment, CH3Cl is a polar molecule.
Uses of CH3Cl
Chloromethane was used as a refrigerant but was discontinued later when it found that the gas has harmful effects.
Methyl chloride is also used as a methylating and chlorinating agent in chemical industries.
This compound is also used as a herbicide and as a local anesthetic as well.
Many industries also use this gas as a catalyst carrier of the polymerization process at low temperatures.
It is also used as an extractant for oils, greases, and resins.