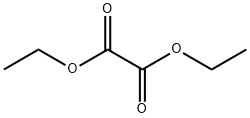
the synthesis of diethyl oxalate
Introduction
Diethyl oxalate is an organic compound with molecular formula C6H10O4. Colorless oily liquid with aromatic smell; Miscible in most organic solvents such as ethanol, ether and ethyl acetate; Relative density: 1.08 (water = 1); 5.04 (air = 1); Stability; Danger sign 14 (with drugs); It is mainly used as solvent, dye intermediate, paint and drug synthesis[1].
Properties
Diethyl oxalate is a colorless oily liquid with aromatic smell. The relative density is 1.0785 (20 / 4 ℃). Melting point - 40.6 ℃. Boiling point 185.4 ℃. Refractive index Nd (20 ℃) 1.4101. Heat of vaporization 284.5j/g. The specific heat capacity is 1.81j / (g ·℃). It is miscible with common solvents such as ethanol, ether and acetone. Slightly soluble in water and gradually decomposed by water. It is mainly used for the intermediates of phenobarbital, bajika malonate, triethylamine and Xinnuoming drugs. It can also be used as the intermediates of Di cotton gelled dyes, plastics, solvents of fibers and spices, and the synthesis of organic compounds. Diethyl oxalate is toxic and easy to hydrolyze into acid and alcohol in the body, resulting in strong corrosiveness and irritation. The oral LD50 of rats was 0.4 ~ 1.6g/kg. Its prominent symptoms are respiratory disorder and muscle fibrillation. Care should be taken to avoid inhalation of vapors and contact with skin. The packaging, storage and transportation of diethyl oxalate are generally packed in galvanized iron drums with a specification of 200kg. Store and transport toxic chemicals according to regulations.
Diethyl oxalate has the general properties of esters. It absorbs moisture in the air and decomposes slowly. It reacts with ammonia to form amide compounds and condenses with acetone to ethyl pyruvate. It is mainly used in the pharmaceutical industry. It is an intermediate of azathioprine, peripheral sulfanilamide, carboxyphenyllipase penicillin, ethoxybenzylpenicillin, chloroquine lactate, thiabendazole, sulfamethoxazole and other drugs. It can be used as an intermediate of plastics, dyes and other products, and as a solvent of cellulose and spices.
Synthesis
Anhydrous oxalic acid and ethanol were esterified in the presence of toluene to produce crude diethyl oxalate. The crude ester is distilled into finished product. Raw material consumption quota: 985kg / T oxalic acid, 744kg / T ethanol (95%) and 73.4kg/t toluene. Another preparation method is to add ethanol, benzene and oxalic acid into the reactor, heat it to 68 ℃, azeotrope reflux dehydration, and take no water out as the end point of the reaction, then recover benzene to obtain crude diethyl oxalate, distill under reduced pressure, and collect 103 ℃ / 6kpa fraction to diethyl oxalate. It is purified by washing with dilute sodium carbonate solution, drying anhydrous potassium carbonate or sodium sulfate and vacuum distillation.
Another preparation method is to add 45g (0.5mol) of anhydrous oxalic acid ① (2), 81g (1.76mol) of anhydrous ethanol, 200ml of benzene and 10ml of concentrated sulfuric acid into the reaction bottle equipped with agitator and water separator. It is heated under stirring and refluxed at 68 ~ 70 ℃ for azeotropic dehydration. After the water is basically evaporated, ethanol and benzene are evaporated. Wash with water after cooling, wash with saturated sodium bicarbonate solution, wash with water, and dry with anhydrous sodium sulfate. Diethyl oxalate (57g) was obtained by atmospheric distillation and collecting the fraction at 182 ~ 184 ℃, with a yield of 78%. ① dehydrate oxalic acid with anhydrous chloroform water until it crystallizes as follows: steam it with anhydrous oxalic acid and inject it into the powder containing carbon. Filter by suction, dry and store in dryer for standby. Anhydrous oxalic acid can also be prepared by drying directly in an oven. In this experiment, a corresponding amount of oxalic acid containing crystal water can also be used, but the reaction time is longer.
Application
Diethyl diacetate is mainly used in the pharmaceutical industry. It is an intermediate of phenobarbital, azathioprine, peripheral sulfonamide, sulfamethoxazole, carboxyphenyl penicillin, ethylpipeoxyampicillin, chloroquine lactate, thiabendazole and other drugs. It is also a plastic accelerator and dye intermediate. It is also used as a solvent for cellulose esters and spices. It is used as acetylene extractant and raw material of dyes, medicine and spices.
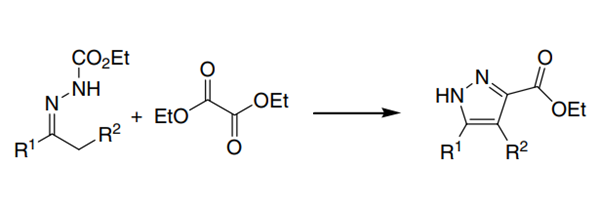
Diethyl acetate is often used as the substrate of nucleophilic reagent α,γ- Dicarbonyl esters, ketone compounds, synthesis of heterocyclic compounds, etc. synthesis α,γ- Dicarbonyl esters can be formed by nucleophilic substitution reaction between ketones and diethyl oxalate under alkaline conditions α,γ- Dicarbonyl ester (formula 1). The dicarbonyl ester often exists in enol structure and can be used to synthesize heterocyclic compounds (formula 2)
Picture 1 Formula 1 and formula 2
Reference
1 Xu G, Li Y, Li Z, et al. Kinetics of the hydrogenation of diethyl oxalate to ethylene glycol[J]. Industrial & engineering chemistry research, 1995, 34(7): 2371-2378.
Related articles And Qustion
See also
Lastest Price from Diethyl oxalate manufacturers

US $10.00/kg2025-04-21
- CAS:
- 95-92-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 95-92-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000mt/year